10.2 Explain why
(a) The angle of contact of mercury with glass is obtuse, while that of water with glass is acute.
(b) Water on a clean glass surface tends to spread out while mercury on the same surface tends to form drops. (Put differently, water wets glass while mercury does not.)
(c) Surface tension of a liquid is independent of the area of the surface
(d) Water with detergent disolved in it should have small angles of contact.
(e) A drop of liquid under no external forces is always spherical in shape
(a) Let a drop of a liquid L be poured on a solid surface S placed in air A. If TSL, and TSA be the surface tensions corresponding to solid-liquid layer, liquid-air layer, and solid-air layer respectively and θ be the angle of contact between the liquid and solid, then
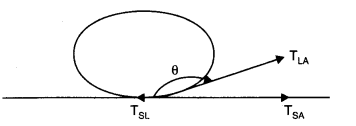
For the mercury-glass interface, TSA< TSL. Therefore, is negative. Thus θ is an obtuse angle. For the water-glass interface, TSA > TSL. Therefore, is positive. Thus, θ is an acute angle.
(b) Water wets glass because the force of cohesion of water is much less than the force of adhesion due to glass. In the case of mercury force of cohesion due to mercury molecules is quite strong as compared to the adhesion force due to glass. So, mercury does not wet glass and tends to create drops.
(c) Surface tension of liquid can be defined as the force acting per unit length on a line drawn tangentially to the liquid surface at rest. Since force is independent of the area of the liquid surface therefore, surface tension is also independent of the area of the liquid surface.
(d) As we know the clothes have narrow pores or spaces which act as capillaries. And the rise of liquid in a capillary tube is directly proportional to cosθ (Here θ is the angle of contact). As θ is small for detergent, therefore cos θ will be large. Due to this, the detergent will penetrate more in the narrow pores of the clothes.
(e) Since, any system tries to remain in a state of minimum energy. In the absence of any external force for a given volume of liquid, its surface area and surface energy is least for a spherical shape. It is due to this reason that a liquid drop, in the absence of an external force is spherical in shape.

© 2026 GoodEd Technologies Pvt. Ltd.