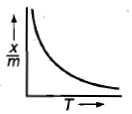
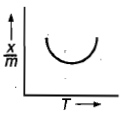
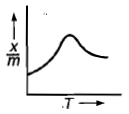
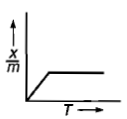
Which plot is the adsorption isobar for chemisorption where X is the amount of gas adsorbed on mass m (at constant pressure) at temperature T
1. 
2. 
3. 
4. 
Adsorption of log(x/m) and log P was found of the following type. This is true when:-
1. n =
2. 1/n = 1
3. P = 0
4. 1/n =
In the langmuir adsorption isotherm, the postulates were:-
(A) Adsorption of a gas molecule at a particular site depends on neighboring sites.
(B) Gases undergoes adsorption behave ideally
(1) Only A is correct
(2) Only B is correct
(3) Both are correct
(4) None of these
According to Freundlich adsorption isotherm, which of the following is correct:-
(1)
(2)
(3)
(4) All are correct for different ranges of pressure
The Langmuir adsorption isotherm is deduced using the assumption:
(1) The adsorption takes place in multilayers
(2) The adsorption sites are equivalent in their ability to adsorb the particles
(3) The heat of adsorption varies with coverage
(4) The adsorbed molecules interact with each other
A plot of log x/m verses log p for the adsorption of a gas on a solid gives a straight line with slope equal to:-
(1) -log k
(2) n
(3) 1/n
(4) log k
Graph between log and log P is a straight line inclined at an angle - 45°. When pressure of 0.5 atm and log k = 0.699, the amount of solute adsorbed per g of adsorbent will be
(1) 1 g/g adsorbent
(2) 1.5 g/g adsorbent
(3) 2.5 g/g adsorbent
(4) 0.25 g/g adsorbent
In an adsorption experiment, a graph between log(x/m) versus log P was found to be linear with a slope of 45. The intercept on the y-axis was found to be 0.3. What will be the value of x/m at a pressure of 3 atm for the Freundlich adsorption isotherm when 1/n remains constant?
[Antilog 0.3 = 2]
1. 2
2. 4
3. 6
4. 5
In the Freundlich adsorption isotherm, the value of 1/n is:
1. Between 0 and 1 in all cases.
2. Between 2 and 4 in all cases.
3. 1 in the case of physical adsorption.
4. 1 in the case of chemisorption.
If x is the amount of adsorbate and m is the amount of adsorbent, which of the following relations is not related to adsorption process?
1.
2.
3.
4.







