A catalyst will not:
| 1. | Increase the forward reaction rate. |
| 2. | Shift the equilibrium to favor the products. |
| 3. | Alter the reaction pathway. |
| 4. | Increase the speed at which equilibrium will be achieved. |
Subtopic: Catalyst |
55%
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Which of the following statements is/are incorrect?
1. i only
2. i and ii only
3. ii and iii only
4. iii only
| (i) | A catalyst lowers the activation energy of a reaction. |
| (ii) | A catalyst allows the same rate of reaction to be achieved at a lower temperature. |
| (iii) | A catalyst mixes with the reactants and increases the overall concentration of reactants in the rate equation. |
2. i and ii only
3. ii and iii only
4. iii only
Subtopic: Catalyst |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Given below are two statements:
Assertion(A): The catalyst can increase the rate constant to a large extent.
Reason(R): By using a suitable catalyst, we can increase the rate of reaction.
1. Both A and R are correct and R is the correct explanation of A.
2. Both A and R are correct but R is not the correct explanation of A.
3. A is true and R is false
4. A is false and R is true
Assertion(A): The catalyst can increase the rate constant to a large extent.
Reason(R): By using a suitable catalyst, we can increase the rate of reaction.
1. Both A and R are correct and R is the correct explanation of A.
2. Both A and R are correct but R is not the correct explanation of A.
3. A is true and R is false
4. A is false and R is true
Subtopic: Catalyst |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Consider the following graph
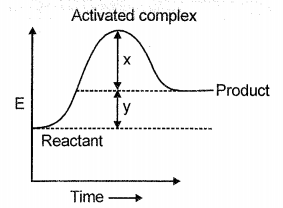
Choose the incorrect statement(s)
1. Reaction is exothermic forward direction i.e., H = -ve
2. Activation energy for forward reaction is x - y
3. Using catalyst energy will decrease H value of the reaction
4. All of these
Subtopic: Catalyst |
77%
Level 2: 60%+
Please attempt this question first.
Hints
Please attempt this question first.


