The total number of resonating structures (excluded the given structure) formed by the given molecule are :
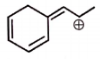
1.
2
2.
3
3.
4
4.
5

The carbocation among the following that doesn't get stabilized by resonance is :
| 1. | 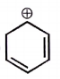 |
2. | 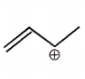 |
| 3. | 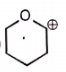 |
4. | 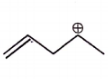 |
The major contributor to the resonance hybrid among the following resonance structures is-
\(\underset{\left(\right. \mathbf{I} \left.\right)}{\left(CH\right)_{3} - \left(CH\right)_{2} - C \overset{\oplus}{H} - O_{\cdot \cdot}^{\cdot \cdot} \left(CH\right)_{3} \underset{}{\leftrightarrow}}\)
\(\underset{\left(\right. \mathbf{II} \left.\right)}{ \left(CH\right)_{3} - \left(CH\right)_{2} - CH = O_{\cdot \cdot}^{\oplus} \left(CH\right)_{3}}\)
1. I
2. II
3. Both have equal contribution
4. They are not resonance structures
The most suitable method used for the separation of 1:1 mixture of ortho and para-nitrophenols is:
| 1. | Chromatography | 2. | Crystallization |
| 3. | Steam distillation | 4. | Sublimation |
The correct statement regarding electrophile is :
| 1. | Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from another electrophile |
| 2. | Electrophiles are generally neutral species and can form a bond by accepting a pair of electrons from a nucleophile |
| 3. | Electrophiles can be either neutral or positively charged species and can form a bond accepting a pair of electrons from a nucleophile |
| 4. | Electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from a nucleophile |
The correct order with respect to –I effect of the substituents is:
(R = alkyl)
1. –NH2 > –OR < –F
2. –NR2 < –OR < –F
3. –NH2 > –OR > –F
4. –NR2 > –OR > –F

| 1. | 4 | 2. | 8 |
| 3. | 12 | 4. | 16 |
In Kjeldahl’s method for estimation of nitrogen present in the soil sample, ammonia evolved from 0.75g of sample neutralized 10ml of 1M H2SO4. The percentage of nitrogen in the soil is:
| 1. | 37.33 | 2. | 45.85 |
| 3. | 25.75 | 4. | 43.13 |
The most stable canonical structure among the given structures is/are :
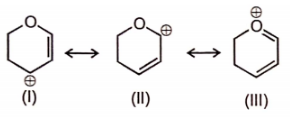
| 1. | I | 2. | II |
| 3. | III | 4. | All are equally stable |
Which structure corresponds to the IUPAC name 3-Ethyl-2-hydroxy-4-methylhex-3-en-5-ynoic acid?
| 1. |  |
2. |  |
| 3. |  |
4. |  |






