| \(\mathrm{(A)}\) |  |
\(\mathrm{(B)}\) | 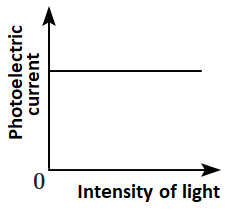 |
| \(\mathrm{(C)}\) |  |
\(\mathrm{(D)}\) |  |
1. \(\mathrm{(A)}\) and \(\mathrm{(D)}\)
2. \(\mathrm{(B)}\) and \(\mathrm{(D)}\)
3. \(\mathrm{(A)}\) only
4. \(\mathrm{(A)}\) and \(\mathrm{(C)}\)
| 1. | \(\mathrm{Na}\) only | 2. | \(\mathrm{Cs}\) only |
| 3. | both \(\mathrm{Na}\) and \(\mathrm{K}\) | 4. | \(\mathrm{K}\) only |
The maximum kinetic energy of the emitted photoelectrons in the photoelectric effect is independent of the:
| 1. | work function of material |
| 2. | intensity of incident radiation |
| 3. | frequency of incident radiation |
| 4. | wavelength of incident radiation |
In a photoelectric experiment, blue light is capable of ejecting a photoelectron from a specific metal while green light is not able to eject a photoelectron. Ejection of photoelectrons is also possible using light of the colour:
| 1. | yellow | 2. | red |
| 3. | violet | 4. | orange |
| 1. | four times | 2. | one-fourth |
| 3. | zero | 4. | doubled |
The work function of the photosensitive material is \(4.0~\text{eV}\). The longest wavelength of light that can cause photoelectric emission from the substance is (approximately):
1. \(3100~\text{nm}\)
2. \(966~\text{nm}\)
3. \(31~\text{nm}\)
4. \(310~\text{nm}\)
1. 1.3 V
2. 0.5 V
3. 2.3 V
4. 1.8 V
A source S1 is producing 1015 photons per sec of wavelength 5000 Å. Another source S2 is producing 1.02×1015 photons per second of wavelength 5100 Å. Then, (power of S2)/(power of S1) is equal to:
1. 1.00
2. 1.02
3. 1.04
4. 0.98
2. 3 X 1016
3. 9 x 1015
4. 3 X 1019

| 1. | Curves \(a\) and \(b\) represent incident radiations of different frequencies and different intensities. |
| 2. | Curves \(a\) and \(b\) represent incident radiation of the same frequency but of different intensities. |
| 3. | Curves \(b\) and \(c\) represent incident radiation of different frequencies and different intensities. |
| 4. | Curves \(b\) and \(c\) represent incident radiations of the same frequency having the same intensity. |






