electrolysed by using inert electrodes. The values of standard electrode potentials in volts
(reduction potentials) are:
\(\begin{aligned} & \mathrm{Ag} / \mathrm{Ag}^{+}=+0.80,2 \mathrm{Hg} / \mathrm{Hg}_2^{++}=+0.79 \\ & \mathrm{Cu} / \mathrm{Cu}^{++}=+0.34, \mathrm{Mg} / \mathrm{Mg}^{++}=-2.37 \end{aligned}\)
With increasing voltage, the sequence of deposition of metals on the cathode will be :
1. Ag, Hg, Cu, Mg
2. Mg, Cu, Hg, Ag
3. Ag, Hg, Cu
4. Cu, Hg, Ag
Consider the following Galvanic cell.
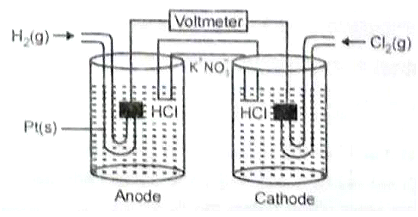
By what value the cell voltage change when concentration of ions in anodic and cathodic compartments both increased by a factor of 10 at 298
1. +0.0591
2. -0.0591
3. -0.1182
4. 0
| Assertion(A): | \(\mathrm{E^0_{cell}}\) = 0 for a chloride ion concentration cell. |
| Reason(R): | For this concentration cell, the equation is given by: \(E_{\mathrm{cell}}=\frac{R T}{n F} \ln \left(\frac{\left[\mathrm{Cl}^{-}\right]_{\mathrm{LHS}}}{\left[\mathrm{Cl}^{-}\right]_{\mathrm{RHS}}}\right) \) |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A) |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
What is the nearest integer value of \(x\) for the Gibbs free energy change at 298 K, expressed as \(x×10^{−1}~kJ mol^{−1}\) for the given reaction.
\(Cu(s)+Sn^{2+}(0.001 M)\rightarrow Cu^{2+}(0.01M)+Sn(s) \)
Given: \(\small{E^\ominus_{Cu^{2+}/Cu}=0.34~V;~E^\ominus_{Sn^{2+}/Sn}=-0.14~V;~F=96500~C~mol^{-1}}\)
1. 873
2. 983
3. 1002
4. 911
| Statement I | For \(\mathrm{Kl},\) molar conductivity increases steeply with dilution. |
| Statement II | For carbonic acid, molar conductivity increases slowly with dilution. |
| 1. | Statement I is incorrect and Statement II is correct. |
| 2. | Both Statement I and Statement II are correct. |
| 3. | Both Statement I and Statement II are incorrect. |
| 4. | Statement I is correct and Statement II is incorrect. |
| List-I | List-II | ||
| (P) |  |
(i) | Conductivity decreases and then increases. |
| (Q) |  |
(ii) | Conductivity decreases and then does not change much. |
| (R) |  |
(iii) | Conductivity increases and then does not change much. |
| (S) |  |
(iv) | Conductivity does not change much and then increases. |
Codes:
| P | Q | R | S | |
| 1. | (iii) | (iv) | (ii) | (i) |
| 2. | (iv) | (iii) | (ii) | (i) |
| 3. | (ii) | (iii) | (iv) | (i) |
| 4. | (i) | (iv) | (iii) | (ii) |
| Assertion (A): | Molar conductivity increases with a decrease in concentration. |
| Reason (R): | For strong electrolytes, \(Λ_m\) increases slowly with dilution and can be represented by the equation: \(\Lambda_m=\Lambda_m^0-A c^{1 / 2}\) |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Value of \(\land_{m}^{0}\) for \(\) \(SrCl_{2}\) (strong electrolyte) in water at 25°C from the data below is:
| Conc. (mol/litre) | 0.25 | 1 |
| \(\land_{m} \Omega^{- 1 } c m^{2 } m o l^{- 1}\) | 260 | 250 |
1. 270 Ω-1 cm2 mol-1
2. 260 Ω-1 cm2 mol-1
3. 250 Ω-1 cm2 mol-1
4. 255 Ω-1 cm2 mol-1
| 1. | \(2.5 \times 10^{-3}\) | 2. | \(2 \times 10^{-3}\) |
| 3. | \(2.5 \times 10^{-4}\) | 4. | \(2 \times 10^{-4}\) |
During electrolysis of conc. H2SO4, perdisulphuric acid (H2S2O8), and O2
form in equimolar amount. The amount of H2 that will form simultaneously will be :
1. Thrice that of O2 in moles.
2. Twice that of O2 in moles.
3. Equal to that of O2 in moles.
4. Half of that of O2 in moles.






