Select Chapter Topics:
The correct stability order of the following species/molecules is :

1.
q > r > p
2.
r > q > p
3.
q > p > r
4.
p > q > r
Subtopic: Aromaticity & Polarity |
54%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Consider the given two statements:
In the light of the above statements, choose the correct answer from the options given below:
| Statement I: |
Methylcyclopentane and hex-3-ene are isomeric compounds.
|
| Statement II: | 1-Aminopentane and N-ethylpropylamine are functional isomers. |
| 1. | Both Statement I and Statement II are false. |
| 2. | Both Statement I and Statement II are true. |
| 3. | Statement I is true but Statement II is false. |
| 4. | Statement I is false but Statement II is true. |
Subtopic: Structural Isomers |
57%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
The correct arrangement of the following carbocations in decreasing order of their stability is:

1. A > B > C > D
2. B > C > A > D
3. C > B > A > D
4. C > A > B > D
Subtopic: Electron Displacement Effects |
54%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
In the given structure, the number of sp and sp2 hybridized carbon atoms present, respectively, are :


| 1. | 3 and 6 | 2. | 3 and 5 |
| 3. | 4 and 6 | 4. | 4 and 5 |
Subtopic: Hybridisation & Structure of Carbon Compounds |
83%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Identify correct statement(s):
Choose the correct answer from the options given below :
1. (A), (C) and (D) only
2. (A), (B) and (E) only
3. (A) only
4. (A) and (C) only
| (A) | —OCH3 and —NHCOCH3 are activating groups. |
| (B) | —CN and —OH are meta-directing groups. |
| (C) | —CN and —SO3H are meta-directing groups. |
| (D) | Activating groups act as ortho – and para-directing groups. |
| (E) | Halides are activating groups. |
1. (A), (C) and (D) only
2. (A), (B) and (E) only
3. (A) only
4. (A) and (C) only
Subtopic: Electron Displacement Effects |
81%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Which one of the carbocations from the following is most stable?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Electron Displacement Effects | Reaction Intermediates ; Preparation & Properties |
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
In the Carius method for estimation of halogens, 180 mg of an organic compound produced 143.5 mg of AgCl. The percentage composition of chlorine in the compound is:
[Given: molar mass in g mol–1 of Ag and Cl are108, 35.5 respectively]
1. 27.91%
2. 46.32%
3. 19.72%
4. 59.67%
[Given: molar mass in g mol–1 of Ag and Cl are108, 35.5 respectively]
1. 27.91%
2. 46.32%
3. 19.72%
4. 59.67%
Subtopic: Quantitative Analysis of Organic Compounds |
80%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
The IUPAC name of the following compound is :
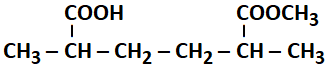
1. 2-Carboxy-5-methoxycarbonylhexane.
2. Methyl-6-carboxy-2,5-dimethylhexanoate.
3. Methyl-5-carboxy-2-methylhexanoate.
4. 6-Methoxycarbonyl-2,5-dimethylhexanoic acid.
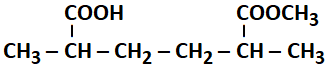
1. 2-Carboxy-5-methoxycarbonylhexane.
2. Methyl-6-carboxy-2,5-dimethylhexanoate.
3. Methyl-5-carboxy-2-methylhexanoate.
4. 6-Methoxycarbonyl-2,5-dimethylhexanoic acid.
Subtopic: Nomenclature |
77%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
The correct structure for the IUPAC name 3-Methylpent-2-enal is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Nomenclature |
85%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Arrange the following in increasing order of their stability:
1. (a) < (b) < (c) < (d)
2. (a) < (d) < (c) < (b)
3. (a) < (c) < (d) < (b)
4. (d) < (c) < (a) < (b)
| a. |  |
b. |  |
| c. |  |
d. |  |
1. (a) < (b) < (c) < (d)
2. (a) < (d) < (c) < (b)
3. (a) < (c) < (d) < (b)
4. (d) < (c) < (a) < (b)
Subtopic: Electron Displacement Effects |
75%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Select Chapter Topics:




