| \(\mathrm{(A)}\) | 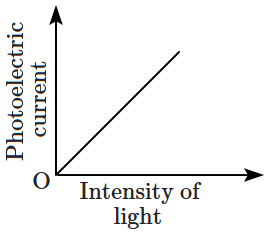 |
\(\mathrm{(B)}\) | 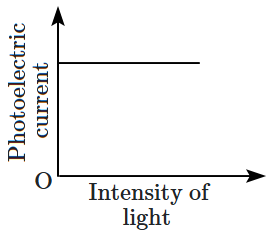 |
| \(\mathrm{(C)}\) | 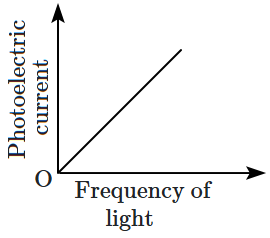 |
\(\mathrm{(D)}\) | 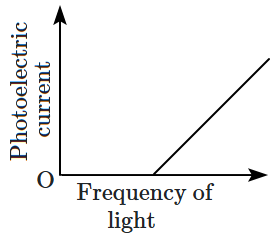 |
2. \(\mathrm{(B)}\) and \(\mathrm{(D)}\)
3. \(\mathrm{(A)}\) only
4. \(\mathrm{(A)}\) and \(\mathrm{(C)}\)
The maximum kinetic energy of the emitted photoelectrons in the photoelectric effect is independent of the:
| 1. | work function of material |
| 2. | intensity of incident radiation |
| 3. | frequency of incident radiation |
| 4. | wavelength of incident radiation |
| 1. | \(\mathrm{Na}\) only | 2. | \(\mathrm{Cs}\) only |
| 3. | both \(\mathrm{Na}\) and \(\mathrm{K}\) | 4. | \(\mathrm{K}\) only |
In a photoelectric experiment, blue light is capable of ejecting a photoelectron from a specific metal while green light is not able to eject a photoelectron. Ejection of photoelectrons is also possible using light of the colour:
| 1. | yellow | 2. | red |
| 3. | violet | 4. | orange |
| 1. | \(V_0 /2\) | 2. | \(V_0 \) |
| 3. | \(4V_0 \) | 4. | \(2V_0 \) |
(take \(h\) as Plank's constant and \(c\) as the velocity of light in free space)
| 1. | \({e}+2\phi \) | 2. | \(2{e}-\phi \) |
| 3. | \({e}-\phi \) | 4. | \({e}+\phi \) |
(take \(h=6.62\times10^{-34}~\text{J s}\) and \(c=3\times10^{8}~\text{ms}^{-1}\))
| 1. | \(4.4~\text{eV}\) | 2. | \(7.103\times10^{-15}~\text{J}\) |
| 3. | \(1.9~\text{eV}\) | 4. | \(4.60~\text{eV}\) |
| 1. | \(2~\text{eV}\) | 2. | \(2~\text{V}\) |
| 3. | \(1.1~\text{V}\) | 4. | \(6.4~\text{V}\) |
When the light of frequency \(2\nu_0\) (where \(\nu_0\) is threshold frequency), is incident on a metal plate, the maximum velocity of electrons emitted is \(v_1.\) When the frequency of the incident radiation is increased to \(5\nu_0,\) the maximum velocity of electrons emitted from the same plate is \(v_2.\) What will be the ratio of \(v_1\) to \(v_2?\)
| 1. | \(1:2\) | 2. | \(1:4\) |
| 3. | \(4:1\) | 4. | \(2:1\) |
Two radiations of photons energies \(1\) eV and \(2.5\) eV, successively illuminate a photosensitive metallic surface of work function \(0.5\) eV. The ratio of the maximum speeds of the emitted electrons is:
1. \(1:2\)
2. \(1:1\)
3. \(1:5\)
4. \(1:4\)






