Which of the following options represents the variation of photoelectric current with the property of light shown on the \(x \text{-}\)axis?
1. \(\mathrm{(A)}\) and \(\mathrm{(D)}\)
2. \(\mathrm{(B)}\) and \(\mathrm{(D)}\)
3. \(\mathrm{(A)}\) only
4. \(\mathrm{(A)}\) and \(\mathrm{(C)}\)
| \(\mathrm{(A)}\) |  |
\(\mathrm{(B)}\) | 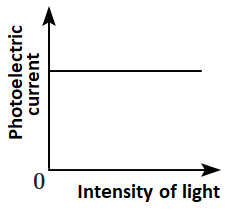 |
| \(\mathrm{(C)}\) |  |
\(\mathrm{(D)}\) |  |
1. \(\mathrm{(A)}\) and \(\mathrm{(D)}\)
2. \(\mathrm{(B)}\) and \(\mathrm{(D)}\)
3. \(\mathrm{(A)}\) only
4. \(\mathrm{(A)}\) and \(\mathrm{(C)}\)
Subtopic: Photoelectric Effect: Experiment |
54%
From NCERT
NEET - 2025
Please attempt this question first.
Hints
Please attempt this question first.
The work functions of Caesium \((\mathrm{Cs}),\) Potassium \((\mathrm{K}),\) and Sodium \((\mathrm{Na})\) are \(2.14~\text{eV},\) \(2.30~\text{eV}\) and \(2.75~\text{eV}\) respectively. If incident electromagnetic radiation has an incident energy of \(2.20~\text{eV},\) which of these photosensitive surfaces may emit photoelectrons?
| 1. | \(\mathrm{Na}\) only | 2. | \(\mathrm{Cs}\) only |
| 3. | both \(\mathrm{Na}\) and \(\mathrm{K}\) | 4. | \(\mathrm{K}\) only |
Subtopic: Photoelectric Effect: Experiment |
68%
From NCERT
NEET - 2023
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
The maximum kinetic energy of the emitted photoelectrons in the photoelectric effect is independent of the:
| 1. | work function of material |
| 2. | intensity of incident radiation |
| 3. | frequency of incident radiation |
| 4. | wavelength of incident radiation |
Subtopic: Photoelectric Effect: Experiment |
80%
From NCERT
NEET - 2023
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
In a photoelectric experiment, blue light is capable of ejecting a photoelectron from a specific metal while green light is not able to eject a photoelectron. Ejection of photoelectrons is also possible using light of the colour:
| 1. | yellow | 2. | red |
| 3. | violet | 4. | orange |
Subtopic: Photoelectric Effect: Experiment |
84%
From NCERT
NEET - 2022
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Light of a frequency of \(1.5\) times the threshold frequency is incident on a photosensitive material. What happens to the photoelectric current when the frequency is cut in half and the intensity is doubled?
| 1. | four times | 2. | one-fourth |
| 3. | zero | 4. | doubled |
Subtopic: Photoelectric Effect: Experiment |
55%
From NCERT
NEET - 2020
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
Links
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
The work function of the photosensitive material is \(4.0~\text{eV}\). The longest wavelength of light that can cause photoelectric emission from the substance is (approximately):
1. \(3100~\text{nm}\)
2. \(966~\text{nm}\)
3. \(31~\text{nm}\)
4. \(310~\text{nm}\)
Subtopic: Photoelectric Effect: Experiment |
75%
From NCERT
NEET - 2019
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
Links
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital




