The coefficient of performance of an ideal refrigerator is 3 which extracts heat from the sink at the rate of 399 J per cycle.
The amount of heat it gives to the room per cycle will be:
1 532 J
2 250 J
3 300 J
4 496 J
If a refrigerator extracts heat 'a' from the cold reservoir and 'b' is the heat released from the hot reservoir, then the work done on the refrigerant (system) is:
1. a + b
2.
3. a
4.
A refrigerator whose coefficient of performance is 5 extracts heat from the cooling chamber at a rate of 250 J per cycle. For refrigeration, the work done per cycle is:
1. 150 J
2. 200 J
3. 100 J
4. 50 J
| Assertion (A): | Carnot engine is most efficient among all heat engines working between the same source and sink. |
| Reason (R): | The efficiency of the heat engine is independent of the nature of the working substance. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false |
An ideal refrigerator has a freezer at a temperature of –13°C . The coefficient of performance of the engine is 5. The temperature of the air (at which heat is rejected) will be
1. 325°C
2. 325K
3. 39°C
4. 320°C
A steam engine delivers \(5.4\times 10^8\) J of work per minute and extracts \(3.6\times 10^9\) J of heat per minute from its boiler. The efficiency of the engine is:
1. \(15\%\)
2. \(18\%\)
3. \(13\%\)
4. \(11\%\)
A refrigerator is to maintain eatables kept inside at \(9^\circ \text{C}.\) If room temperature is \(36^\circ \text{C},\) the coefficient of performance is:
1. \(9.3\)
2. \(12.4\)
3. \(11.2\)
4. \(10.4\)
Consider a heat engine as shown in the figure. are heat added to and heat taken from respectively, in one cycle of the engine. W is the mechanical work done on the engine.
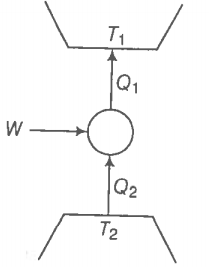
If W > 0, then possibilities are:
Choose the correct alternatives:
1. (b, c)
2. (a, d)
3. (b, d)
4. (a, c)
An engine has an efficiency of . When the temperature of the sink is reduced by , its efficiency is doubled. the temperature of the source is:
1. 124oC
2. 37oC
3. 62oC
4. 99oC
A Carnot engine, having an efficiency of = as a heat engine, is used as a refrigerator. If the work done on the system is \(10\) J, the amount of energy absorbed from the reservoir at a lower temperature is:
1. \(100\) J
2. \(99\) J
3. \(90\) J
4. \(1\) J


