Which of the following compounds produces a pink colour when combined with Schiff's reagent?
1. CH3CHO
2. CH3CH2COOH
3. C3H5COOH
4. C6H5COC6H5
Given the reaction:

Which of the following statements is correct?
1. (b) has a lesser stability
2. (b) is more volatile than (a)
3. (a) is more volatile than (b)
4. (a) forms higher yields at a lower temperature
The product in the below mentioned reaction is
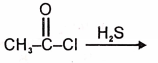
| 1. |  |
2. | 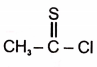 |
| 3. | 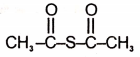 |
4. | 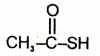 |
The products A and B in the below mentioned reaction are, respectively:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The values of some of the acids are given below:
| pKa |  |
-0.6 |  |
4.8 | \(\overset{+}{N}H_4\) | 9.4 |
| pKa | HI | -10.0 | |
25 | \(H_2S\) | 7.0 |
The correct order of leaving tendency of their conjugate bases is-
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Reaction equilibrium will shift towards the right in:
| 1. | 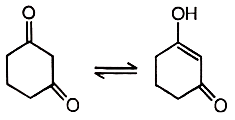 |
2. | 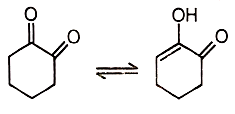 |
| 3. | 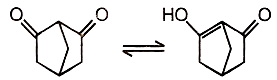 |
4. | 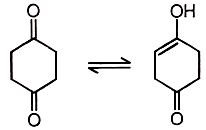 |
The Wolf Kishner's reduction cannot be applied to:
| 1. |  |
2. |  |
| 3. |  |
4. |  |

1. Br2/KOH, H2/Pt, CH3COOH
2. P2O5/heat, H2/Pt, (CH3CO2)2O
3. (CH3CO)2O, H2/Pt, Br2/KOH
4. Br2/KOH, CH3COCl
| 1. | Phenol | 2. | Benzene |
| 3. | Salicylic acid | 4. | Soda ash |
Which of the following acids is the strongest?
1. CH3COOH
2. C6H5COOH
3. C6H5OH
4. C2H5COOH




