The decomposition of hydrocarbons follows the equation: k = (4.5 × 1011s–1) e-28000K/T
The activation energy (Ea) for the reaction would be:
1.
232.79 kJ mol-1
2.
245.86 kJ mol-1
3.
126.12 kJ mol-1
4.
242.51 kJ mol-1
The role of a catalyst is to change:
1. Gibbs energy of the reaction
2. Enthalpy of reaction
3. The activation energy of the reaction
4. Equilibrium constant
The correct statement based on the graph below is:
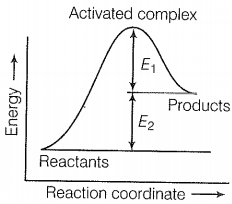
| 1. | The activation energy of the forward reaction is E1 + E2 and the product is less stable than reactant. |
| 2. | The activation energy of the forward reaction is E1 + E2 and the product is more stable than the reactant. |
| 3. | The activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than the product. |
| 4. | The activation energy of the backward reaction is E1 and the product is more stable than reactant. |
Consider the first-order gas-phase decomposition reaction given below.
A(g) → B(g) + C(g)
The initial pressure of the system before the decomposition of A was . After the lapse of time t, the total pressure of the system increased by X units and became . The rate constant k for the reaction is:
1.
2.
3.
4.
The correct graphical representation of relation between ln k and 1/T is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
True statement among the following is:
| 1. | The rate of a reaction decreases with the passage of time as the concentration of reactants decreases. |
| 2. | The rate of a reaction is the same at any time during the reaction. |
| 3. | The rate of a reaction is independent of temperature change. |
| 4. | The rate of a reaction decreases with an increase in the concentration of the reactants. |
The correct expression for the rate of reaction given below is:
\(5 \mathrm{Br}^{-}(\mathrm{aq})+\mathrm{BrO}_3^{-}(\mathrm{aq})+6 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow 3 \mathrm{Br}_2(\mathrm{aq})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l})\)
| 1. | \(\frac{\Delta\left[B r^{-}\right]}{\Delta t}=5 \frac{\Delta\left[H^{+}\right]}{\Delta t} \) | 2. | \(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=\frac{6}{5} \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t} \) |
| 3. | \(\frac{\Delta[\mathrm{Br^-}]}{\Delta t}=\frac{5}{6} \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t} \) | 4. | \(\frac{\Delta\left[\mathrm{Br}^{-}\right]}{\Delta t}=6 \frac{\Delta\left[\mathrm{H}^{+}\right]}{\Delta t}\) |
The correct graphical representation of first-order reaction is:
| (a) |  |
(b) |  |
| (c) |  |
(d) |  |
| 1. | (a) and (b) | 2. | (b) and (c) |
| 3. | (c) and (d) | 4. | (a) and (d) |
Match the graph given in Column I with the order of reaction given in Column II.
More than one item in Column I may be linked to the same item in Column II:
| Column I | Column II | ||
| (a) |  |
(a) | 1st order |
| (b) |  |
(b) | Zero order |
| (c) |  |
||
| (d) |  |
||
| (i) | (ii) | (iii) | (iv) | |
| 1. | (a) | (b) | (a) | (b) |
| 2. | (a) | (b) | (b) | (a) |
| 3. | (a) | (a) | (b) | (b) |
| 4. | (b) | (b) | (a) | (a) |
| Column I | Column II | ||
| A. | Catalyst alters the rate of reaction | 1. | Proper orientation is not there always |
| B. | 2. | By lowering the activation energy | |
| C. | Energetically favorable reactions are sometimes slow | 3. | Total probability is one |
| D. | Area under the Maxwell-Boltzmann curve is constant | 4. | Refers to the fraction of molecules with energy equal to or greater than the activation energy |
Codes
| A | B | C | D | |
| 1. | 2 | 4 | 1 | 3 |
| 2. | 3 | 1 | 4 | 2 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 3 | 4 | 1 | 2 |


