For the reaction, 2A → B, rates= k[A]2. If the concentration of reactant is doubled, then the:
| (a) | rate of reaction will be doubled. |
| (b) | rate constant will remain unchanged, however rate of reaction is directly proportional to the rate constant. |
| (c) | rate constant will change since the rate of reaction and rate constant are directly proportional to each other. |
| (d) | rate of reaction will increase by four times. |
Identify the set of correct statements & choose the correct answer from the options given below:
| 1. | (a) and (c) only | 2. | (a) and (b) only |
| 3. | (b) and (d) only | 4. | (c) and (d) only |
At high pressure the following reaction is zero order-
The correct statements among the following is:
| a. | Rate of reaction = Rate constant |
| b. | Rate of the reaction depends on the concentration of ammonia |
| c. | Rate of decomposition of ammonia will remain constant until ammonia disappears completely |
| d. | Further increase in pressure will change the rate of reaction |
| 1. | (a, b, c) | 2. | (b, c, d) |
| 3. | (a, c, d) | 4. | (a, b, d) |
A graph of volume of hydrogen released vs time for the reaction between zinc and dil. HCl is given in the graph below.
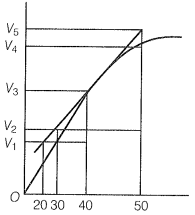
The correct statement among the following based on the graph given above is:
Consider the reaction AB. The concentration of both the reactant and the product varies exponentially with time.
The graph that accurately depicts how reactant and product concentrations change with time is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Match the items in Column I with Column II:
| Column I | Column II |
| A. Diamond to graphite conversion | 1. Short interval of time |
| B. Instantaneous rate | 2. Ordinarily rate of conversion is imperceptible |
| C. Average rate | 3. Long duration of time |
Codes
| A | B | C | |
| 1. | 2 | 1 | 3 |
| 2. | 1 | 2 | 3 |
| 3. | 3 | 2 | 1 |
| 4. | 1 | 3 | 2 |
Based on the graph below, the average rate of reaction will be:
1. \(\frac{[R_{2}]-[R_{1}]}{t_{2}-t_{1}}\)
2. \(-(\frac{[R_{2}]-[R_{1}]}{t_{2}-t_{1}})\)
3. \(\frac{[R_{2}]}{t_{2}}\)
4. \(-(\frac{[R_{1}]-[R_{2}]}{t_{2}-t_{1}})\)
Consider the following graph:
The instantaneous rate of reaction at t = 600 sec will be:





