A first-order reaction's 10 percent completion time at 298 K is the same as its 25 percent completion time at 308 K. The value of will be:
1.
2.
3.
4.
The rate constant for a first-order reaction is . The time required to reduce the initial concentration of the reactant to its 1/16 value is-
1.
2.
3.
4.
A first-order reaction takes 40 min for 30% decomposition. Half life of the reaction is-
1. 55.9 min
2. 77.9 min
3. 63.9 min
4. 80.9 min
If the concentration of the reactant is made twice, the new rate of reaction for the second order reaction would be-
1. 2 times
2. 4 times
3. 3 times
4. No change in the rate of the reaction
Consider the following rate expression.
The order of reaction and dimension of the rate constant are, respectively-
1. ; k =
2. 3; k =
3. ; k =
4. ; k =
Given that . The dimension of the rate constant in the given rate law is -
1.
2.
3.
4.
For a first-order reaction, the relationship between time required for 99% completion to the time required for the completion of 90% of the reaction would be :
For the reaction , rate = with k = and . The initial rate of the reaction will be :
1. 0.04 mol
2. 8
3. 8
4. 8 mol
For the decomposition of azoisopropane to hexane and nitrogen at 543 K, the following data was obtained:
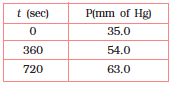
The rate constant of the above reaction would be -
1. 1.21 x 10-3 s-1
2. 2.21 x 10-3 s-1
3. 3.21 x 10-3 s-1
4. 4.21 x 10-3 s-1
During a nuclear explosion, one of the products is 90Sr with a half-life of 28.1 years. If 1µg of 90Sr was absorbed in the bones of a newly born baby instead of calcium, the amount of 90Sr that will remain after 10 years in the now grown up child would be -
(Given ,antilog(0.108)=1.28)
1. 0.227 µg
2. 0.781 µg
3. 7.81 µg
4. 2.27 µg


