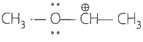Given below are two statements:
Assertion (A):
Pent-1-ene and pent-2-ene are position isomers.
Reason (R):
Position isomers differ in the position of a functional group or a substituent.
1.
Both (A) and (R) are true and (R) is the correct explanation of (A).
2.
Both (A) and (R) are true but (R) is not the correct explanation of (A).
3.
(A) is true but (R) is false.
4.
(A) is false but (R) is true.
Given below are two statements:
| Assertion (A): | All the carbon atoms in H2C = C = CH2 are sp2-hybridised. |
| Reason (R): | In this molecule, all the carbon atoms are attached to each other by double bonds. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
Given below are two statements:
| Assertion (A): | Sulphur present in an organic compound can be estimated quantitatively by the Carius method. |
| Reason (R): | Sulphur is separated easily from other atoms in the molecule and gets precipitated as a light yellow solid. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
Given below are two statements:
| Assertion (A): | Components of a mixture of red and blue inks can be separated by distributing the components between stationary and mobile phases in paper chromatography. |
| Reason (R): | The colored components of inks migrate at different rates because paper selectively retains different components according to the difference in their partition between the two phases. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
Given below are two statements:
| Assertion (A): | Energy of resonance hybrid is equal to the average of energies of all canonical forms. |
| Reason (R): | Resonance hybrid cannot be presented by a single structure. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
Given below are two statements:
| Assertion (A): | Simple distillation can help in separating a mixture of propan-1-ol (boiling point 97 ° C ) and propanone (boiling point 56 ° C ). |
| Reason (R): | Liquids with a difference of more than 20 ° C in their boiling points can be separated by simple distillation. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
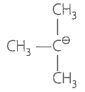
Match the ions given in Column I with their nature-given in Column II.
|
Column I |
Column II |
|
A. |
1. Stable due to resonance |
|
B. |
2. Destabilised due to inductive effect |
|
C. |
3. Stabilised by hyperconjugation. |
|
D. |
4. A secondary carbocation |
Codes
| Options: | A | B | C | D |
| 1. | 1,2 | 2 | 2 | 3,4 |
| 2. | 1 | 2,4 | 3 | 4 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 1 | 3 | 2 |
Match the intermediates given in Column I with their probable structure in Column II.
|
Column I |
Column II |
|
A. Free radical |
1. Trigonal planar |
|
B. Carbocation |
2. Pyramidal |
|
C. Carbanion |
3. Linear |
Codes
| Options: | A | B | C |
| 1. | 1 | 1 | 2 |
| 2. | 1 | 2 | 3 |
| 3. | 3 | 1 | 2 |
| 4. | 2 | 1 | 3 |
Match the terms mentioned in Column I with the terms in Column II.
|
Column I |
Column II |
|
A. Carbocation |
1. Species that can receive a pair of electrons |
|
B. Nucleophile |
2. Conjunction of electrons of bond with the empty p-orbital present in adjacent positively charged carbon |
|
C. Hyperconjugation |
3. hybridised carbon with empty p-orbital |
|
D. Electrophile |
4. Species that can donate a pair of electrons |
Codes
| Options: | A | B | C | D |
| 1. | 3 | 4 | 2 | 1 |
| 2. | 1 | 2 | 3 | 4 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 1 | 3 | 2 |
Match the type of mixture of compounds in Column I with the technique of separation/purification given in column II.
| Column I | Column II |
| A. Two solids which have different solubilities in a solvent and which do not undergo a reaction when dissolved in it | 1. Steam distillation |
| B. Liquid that decomposes at its boiling point | 2. Fractional distillation |
| C. Steam volatile liquid | 3. Crystallisation |
| D. Two liquids that have boiling points close to each other | 4. Distillation under reduced pressure |
Codes
| A | B | C | D | |
| 1. | 3 | 4 | 1 | 2 |
| 2. | 1 | 2 | 3 | 4 |
| 3. | 1 | 4 | 3 | 2 |
| 4. | 4 | 1 | 3 | 2 |