Given below are two statements:
| Assertion (A): | Boric acid is considered a weak acid. |
| Reason (R): | Boric acid is not able to release H+ ions on its own. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
Subtopic: Compounds of Boron- Preparations, Properties & Uses |
From NCERT
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
Hints
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
Incorrect statements among the following are:
1. B and E only
2. B, C and E only
3. A and D only
4. B and D only
| A. | BeSO4 and MgSO4 are insoluble in water. |
| B. | Magnesium is the central metal atom in chlorophyll. |
| C. | Pb4+ acts as an oxidizing agent. |
| D. | The study of only emission, not absorption spectra is referred to as spectroscopy. |
| E. | Diborane is prepared by treating BF3 with LiAlH4 |
1. B and E only
2. B, C and E only
3. A and D only
4. B and D only
Subtopic: Compounds of Boron- Preparations, Properties & Uses | Properties of Glass, Pb & Sn compounds |
58%
From NCERT
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
Hints
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
Which of the following statements are correct?
(Answer on the basis of the given figure.)
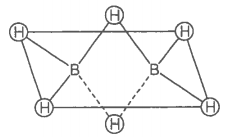
| a. | The two bridged hydrogen atoms and the two boron atoms lie in one plane |
| b. | Out of six B-H bonds, two bonds can be described in terms of 3-center-2-electron bonds |
| c. | Out of six B-H bonds, four B-H bonds can be described in terms of 3 center 2 electron bonds |
| d. | The four-terminal B-H bonds are two center-two electron angular bonds |
Choose the correct option
1. (a, b, c)
2. (b, c, d)
3. (a, c, d)
4. (a, b, d)
Subtopic: Compounds of Boron- Preparations, Properties & Uses |
61%
From NCERT
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh
Hints
To view explanation, please take trial in the course.
NEET 2023 - Target Batch - Aryan Raj Singh


