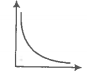
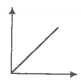
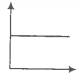
The variation of pressure with the volume of the gas at different temperatures can be
graphically represented as per the following graph:

If the temperature increases from 200K to 400K (at constant pressure), the volume of a
gas would-
1. Increase
2. Decrease
3. Remain constant
4. 1st decreases then increases
graphically represented as per the following graph:

gas would-
A plot of pressure versus for a gas at constant temperature is given below:
The relation between is-
The variation of vapour pressure of different liquids with temperature is shown below:
Boiling points of liquids A and B are, respectively
1. 311 K and 374 K
2. 298 K and 353 K
3. 307 K and 337 K
4. Cannot be determined from the graph given above
Consider the following graph:
The curve that represents CO2 gas is -
1. X
2. Y
3. Both X and Y
4. Cannot predict from the graph
Pressure versus volume graph for a real gas and an ideal gas is shown below:
The correct statement among the following is -
1. Real gases show a large deviation from ideal behaviour at low pressure.
2. Real gases show a very small deviation from ideal behaviour at low pressure.
3. Real gases do not behave ideally under any conditions at low pressure.
4. None of the above statements is correct
The plots of different values of pressure versus temperature are shown in the given figure. The correct order of volume for the following plot is-
1. V4 < V3 < V2 < V1
2. V4 > V3 < V2 > V1
3. V4 > V3 > V2 > V1
4. V4 > V3 > V2 < V1
Match the following graphs of an ideal gas with their coordinates.
|
Graphical representation |
X and Y coordinates |
|
A. |
1. pV vs V |
|
B. |
2. p vs V |
|
C. |
3. p vs |
Codes
A B C
(1) 2 3 1
(2) 1 2 3
(3) 3 2 1
(4) 1 3 1
The correct statements among the following regarding gaseous state is -
| (a) | Complete order of molecules |
| (b) | Complete disorder of molecules |
| (c) | Random motion of molecules |
| (d) | Fixed position of molecules |
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, d)
Given below are two statements:
| Assertion (A): | Liquids tend to have a maximum number of molecules at their surface. |
| Reason (R): | Small liquid drops have a spherical shape. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |
Given below are two statements:
| Assertion (A): | At the critical temperature, the liquid passes into a gaseous state imperceptible and continuously. |
| Reason (R): | The density of the liquid and gaseous phase is equal to critical temperature. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |