The decreasing order of ionic character of the N-H, F-H, C-H, and O-H, is :
1.
N-H > F-H > C-H > O-H
2.
F-H > N-H > C-H > O-H
3.
O-H > C-H > F-H > N-H
4.
F-H > O-H > N-H > C-H
The right order of increase in the ionic character of the molecules,
LiF, K2O, N2, SO2, ClF3 is :
1. N2 < SO2 < ClF3 < K2O < LiF
2. N2 > SO2 > ClF3 > K2O < LiF
3. N2 > SO2 > K2O > ClF3 > LiF
4. LiF > K2O < ClF3 > SO2 > N2
The number of (i) sp2 hybridized carbon atoms and (ii) bonds are present in the following compound are:
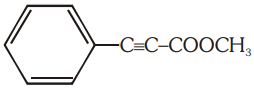
1. 7, 5
2. 8, 6
3. 7, 6
4. 8, 5
From the perspective of molecular orbital theory, which statement is false?
1. is not a stable molecule.
2. is not stable but is expected to exist.
3. Bond strength of is maximum amongst the homonuclear diatomic molecules belonging to the second period.
4. The order of energies of molecular orbitals in molecule is:
Combination of atoms A and B that forms an anti-bonding molecular orbital is :
1.
2.
3.
4.
In , formal charge on every oxygen atom and P-O bond order respectively are:
1. -0.75 and 1.25
2. -0.5 and 2
3. 1 and 1.5
4. -0.75 and 2
In a regular octahedral molecule, MX6, the number of X–M–X bonds at 180º is:
| 1. | Two | 2. | Six |
| 3. | Four | 4. | Three |
In NO3– ion, the number of bond pair and lone pair of electrons on nitrogen atom are respectively:
| 1. | 2, 2 | 2. | 3, 1 |
| 3. | 1, 3 | 4. | 4, 0 |
The incorrect statements among the following are-
| (a) | NaCl being an ionic compound is a good conductor of electricity in the solid-state |
| (b) | In canonical structure, there is a difference in the arrangement of atoms |
| (c) | Hybrid orbitals form stronger bonds than pure orbitals |
| (d) | VSEPR theory can explain the square planar geometry of XeF4 |
| 1. | (a) and (b) only | 2. | (b) and (c) only |
| 3. | (c) and (d) only | 4. | (b) and (d) only |
A pair in which both species are not likely to exist is:
| 1. | \(H^+_2,He^{2-}_2\) | 2. | \(H^-_2,He^{2+}_2\) |
| 3. | \(H^{2+}_2,He_2\) | 4. | \(H^-_2,He^{2+}_2\) |


