The number of bonds and bonds in the following structure is :
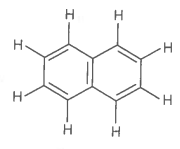
| 1. | 6, 19 | 2. | 4, 20 |
| 3. | 5, 19 | 4. | 5, 20 |
The total number of possible resonance forms for the nitrate ion, () is:
| 1. | 1 | 2. | 2 |
| 3. | 3 | 4. | 4 |
The most preferred structure among the following with the lowest energy for SO3 is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
An incorrect statement regarding resonating structures is :
| 1. | The contributing structure must have the same number of unpaired electrons. |
| 2. | The contributing structures should have similar energies. |
| 3. | The resonance hybrid should have a higher energy than any of the contributing structures. |
| 4. | None of the above. |
can be represented by structures 1 and 2 shown below.
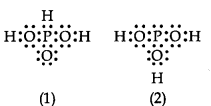
These two structures cannot be taken as the canonical forms of the resonance hybrid, because :
1. The positions of the atoms have changed.
2. The positions of the atoms are constant.
3. H3PO3 does not show resonance.
4. Two hydrogen atoms are missing.
The correct order of 'SO' bond length is:
| 1. | \(\mathrm{SO}_3^{2-}>\mathrm{SO}_4^{2-}>\mathrm{SO}_3>\mathrm{SO}_2 \) |
| 2. | \(\mathrm{SO}_3^{2-}>\mathrm{SO}_4^{2-}>\mathrm{SO}_2>\mathrm{SO}_3 \) |
| 3. | \(\mathrm{SO}_4^{2-}>\mathrm{SO}_3^{2-}>\mathrm{SO}_2>\mathrm{SO}_3 \) |
| 4. | \(\mathrm{SO}_4^{2-}>\mathrm{SO}_3^{2-}>\mathrm{SO}_3>\mathrm{SO}_2\) |
Which of the following has a maximum 'Cl-O' bond order?
1.
2.
3.
4.
Mg2C3 reacts with water forming propyne . The number of sigma and pie bonds in \(C_{3}^{4-}\)is-
1. Two sigma and two pi bonds.
2. Three sigma and one pi bonds.
3. Two sigma and one pi bonds.
4. Two sigma and three pi bonds.


