can be represented by structures 1 and 2 shown below.
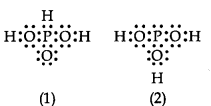
These two structures cannot be taken as the canonical forms of the resonance hybrid, because :
| 1. | The positions of the atoms have changed.
|
| 2. | The positions of the atoms are constant.
|
| 3. | H3PO3 does not show resonance.
|
| 4. | Two hydrogen atoms are missing. |
Subtopic: Resonance & Nature of Compounds |
62%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
The number of resonating structures exhibited by \(CO^{2-}_3\) ion are :
1. 2
2. 3
3. 4
4. 5
Subtopic: Resonance & Nature of Compounds |
79%
From NCERT
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
Hints
To view explanation, please take trial in the course.
NEET 2025 - Target Batch
