The alkene among the following that has the smallest heat of hydrogenation is-
1.
2.
3.
4.
The correct order of relative rates of hydrogenation of alkenes is:
1. Ethylene > propene > 2-butene > 2-methyl-2-butene
2. 2-methyl-2-butene > 2-butene > Propene > Ethylene
3. 2-butene > propene > ethylene > 2-methyl-2-butene
4. Propene > 2-butene > ethylene > 2-methyl-2-butene
A compound that gives an optically inactive compound on treatment with H2(2 moles)/ Pt is-
1. 3-Methyl-1-pentyne
2. 4-Methyl-1-hexyne
3. 3-Methyl-1-heptyne
4. None of the above
Hydrocarbon (A) reacts with bromine by substitution reaction to form an alkyl bromide B.
B undergoes the Wurtz reaction to give a gaseous hydrocarbon containing less than four carbon atoms.
The formula of (A) is:
1.
2.
3.
4.
The compound undergoes the following reactions
C
The product 'C' is-
1. m–Bromotoluene
2. o–Bromotoluene
3. 3–Bromo–2,4,6–trichlorotoluene
4. p–Bromotoluene
The most probable mechanism for the above reaction is-
1. E1
2. E2
3.
4. elimination
The main product of the following reaction will be-
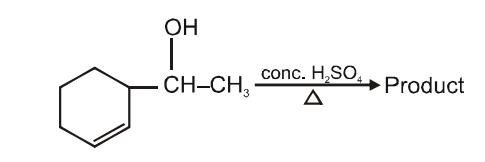
| 1. |  |
2. |  |
| 3. |  |
4. |  |
3-Phenylpropene on reaction with HBr gives (as a major product)-
1.
2.
3.
4.
Anti-Markovnikov’s addition of HBr is not observed in-
| 1. | Propene | 2. | But-1-ene |
| 3. | But-2-ene | 4. | Pent-2-ene |
The reaction of propene with HOCl proceeds via the addition of:
| 1. | H+ in the first step | 2. | Cl+ in the first step |
| 3. | OH- in the first step | 4. | Cl+ and OH- in a single step |




