A closed vessel contains equal number of oxygen and hydrogen molecules. Consider the following statements:
1. The average speed of hydrogen molecules is greater.
2. The two gases have different average energies.
3. Hydrogen molecules strike the walls more often.
4. Weight of hydrogen is th of the weight of oxygen.
Wrong statements are –
(A) 1 and 2
(B) 2 and 3
(C) 1 and 3
(D) 2 and 4
A: At Boyle's temperature, the compressibility factor of gas approaches unity.
R: At Boyle's temperature the real gas obeys ideal gas law.
1. Both assertion & reason are true and the reason is the correct explanation of the assertion.
2. Both assertion & reason are true but the reason is not the correct explanation of the assertion.
3. Assertion is a true statement but reason is false.
4. Both assertion and reason are false statements.
Which of the following statement(s) are correct:
(1) A plot of log KP versus 1/T is linear
(2) A plot of log (X) versus time is linear for a first-order reaction '
(3) A plot of log P versus 1/T is linear at constant volume
(4) A plot of P versus 1/V is linear at a constant temperature.
(1) 1, 2
(2) 2, 4
(3) 2, 3
(4) 1, 4
| Assertion (A) | Critical temperatures for carbon dioxide and methane are 31.1 °C and –81.9 °C respectively. |
| Reason (R) | The intermolecular forces of attraction are stronger in CO2 than CH4 |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | Both (A) and (R) are false. |
A plot of volume versus temperature (T) for a gas at constant pressure is a straight line passing through the origin. The plots at different values of pressure are shown in the figure given below.
The correct order of pressure is -
Temperature (K)
1.
2.
3.
4.
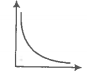
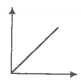
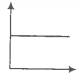
In the given figure, the curve representing an ideal gas is-
1. B only
2. C and D only
3. E and F only
4. A and B only
Match the graphs between the following variables with their names.
|
Graphs |
Names |
|
A. Pressure vs temperature graph at constant molar volume |
1. Isotherms |
|
B. Pressure vs |
2. Constant temperature Curve |
|
C. Volume vs temperature graph at constant pressure. |
3. Isochores |
|
4. Isobars |
Codes
| Options: | A | B | C |
| 1. | 3 | 1 | 4 |
| 2. | 1 | 2 | 3 |
| 3. | 5 | 4 | 3 |
| 4. | 4 | 5 | 3 |
Match the following gas law with the equation representing them.
|
A. Boyle’s law |
1. at constant T and p |
|
B. Charle’s law |
2. …constant T,V |
|
C. Dalton’s law |
3. constant |
|
D. Avogadro’s law |
4. at constant n and p |
|
5. at constant n and T |
Codes
| Options: | A | B | C | D |
| 1. | 5 | 4 | 2 | 1 |
| 2. | 3 | 4 | 2 | 1 |
| 3. | 5 | 4 | 3 | 2 |
| 4. | 4 | 5 | 3 | 2 |
Match the following graphs of an ideal gas with their coordinates.
|
Graphical representation |
X and Y coordinates |
|
A. |
1. pV vs V |
|
B. |
2. p vs V |
|
C. |
3. p vs |
Codes
| Options: | A | B | C |
| 1. | 2 | 3 | 1 |
| 2. | 1 | 2 | 3 |
| 3. | 3 | 2 | 1 |
| 4. | 1 | 3 | 1 |
Given below are two statements:
| Assertion (A): | Three states of matter are the result of a balance between intermolecular forces and the thermal energy of the molecules. |
| Reason (R): | Intermolecular forces tend to keep the molecules together but the thermal energy of molecules tends to keep them apart. |
| 1. | Both (A) and (R) are true and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are true but (R) is not the correct explanation of (A). |
| 3. | (A) is true but (R) is false. |
| 4. | (A) is false but (R) is true. |