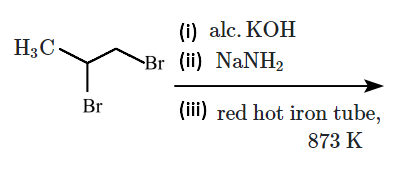Select Chapter Topics:
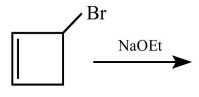
For the given reaction:

'P' is:
1.

2.

3.

4.





Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
Level 3: 35%-60%
NEET - 2024
Hints
Which of the following statements are incorrect in relation to the halogenation of alkane?
| a. | The reactivity of chlorine is less than that of bromine towards alkanes. |
| b. | For the photochemical chlorination of methane, Cl is formed in the slowest step. |
| c. | Free radicals are pyramidal intermediates, stabilized by hyperconjugation and resonance. |
| d. | Bromine has much higher regioselectivity than chlorine in abstracting 3° hydrogen. |
| 1. | a, c, d | 2. | a, b, c |
| 3. | c, d, b | 4. | None of the above |
Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Which of the following reactions gives a meso-compound as the main product?
1. a, b
2. b, c
3. c, d
4. None of the above
| a. |  |
| b. | 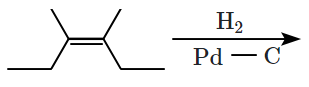 |
| c. |  |
| d. |  |
1. a, b
2. b, c
3. c, d
4. None of the above
Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.

The major product (F) of the above-given reaction sequence is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aromatic Hydrocarbons - Reactions & Mechanism |
Level 3: 35%-60%
JEE
Please attempt this question first.
Hints
Please attempt this question first.
Subtopic: Aromatic Hydrocarbons - Nomenclature, Isomerism & Huckel's Rule |
59%
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Cyclohexene on ozonolysis by reaction with zinc dust and water gives compound E.
Compound E on further treatment with aqueous \(KOH\) yields compound F.
Compound F is:
Compound E on further treatment with aqueous \(KOH\) yields compound F.
Compound F is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aliphatic Hydrocarbon - Methods of Preparation |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Why does the addition of \(\text{HI}\) in the presence of a peroxide catalyst not follow anti-Markovnikov's rule?
| 1. | \(HI\) is a strong reducing agent. |
| 2. | The \(\text{H–I}\) bond is too strong to be broken homolytically. |
| 3. | The \(I\) atom combines with \(H\) atom to give back \(HI.\) |
| 4. | The iodine atom is not reactive enough to add across a double bond. |
Subtopic: Aliphatic Hydrocarbon - Methods of Preparation |
Level 4: Below 35%
Please attempt this question first.
Hints
Please attempt this question first.
Find out the correct statement:
1. Only B is correct.
2. A and C.
3. B and C.
4. Only A is correct.
| A. | The number of possible theoretical conformations of ethane is 6. |
| B. | In the conformations of ethane, the staggered form has the least torsional strain, and the eclipsed form has the maximum torsional strain. |
| C. | Hückel Rule: The presence of (4n + 2) π electrons in the ring where n is an integer (n = 1, 2, ......). |
2. A and C.
3. B and C.
4. Only A is correct.
Subtopic: Aliphatic Hydrocarbon- Physical Properties | Aromatic Hydrocarbons - Nomenclature, Isomerism & Huckel's Rule |
Level 4: Below 35%
Please attempt this question first.
Hints
Please attempt this question first.
Among the following, the compound which contains the fewest tertiary carbons is:
| 1. | \((CH_3)_3C(CH_2)_2CH_3~~~~~~~~~~~~~\) |
| 2. | 4-isobutyl heptane |
| 3. |  |
| 4. |  |
Subtopic: Aliphatic Hydrocarbon- Physical Properties |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Consider the following statements:
The correct statements is/are:
| a. | Benzene hexachloride is an aromatic compound. |
| b. | \(-NHCOCH_3\) is meta directing group towards aromatic electrophilic substitution reaction. |
| c. | In an arenium ion, one of the carbon is sp3 hybridised. |
| 1. | (a) and (b) only | 2. | (a) and (c) only |
| 3. | (c) only | 4. | (a), (b) and (c) |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Select Chapter Topics: