On the basis of mode of formation, polymers can be
classified as:
1. Addition polymers only.
2. Condensation polymers only.
3. Co-polymers.
4. Both addition and condensation polymers.
1. Addition polymers only.
2. Condensation polymers only.
3. Co-polymers.
4. Both addition and condensation polymers.
Low-density polythene is prepared by -
1. Free-radical polymerization.
2. Cationic polymerization.
3. Anionic polymerization.
4. Ziegler-Natta polymerization.
The copolymer from the given options is-
1. Nylon 6, 6
2. Polyethylene
3. PMMA
4. Nylon-6
An example of elastomer among the following is:
1. Dacron
2. Melamine
3. Vulcanized rubber
4. Polystyrene
The thermoplastic polymer among the following is -
1. Bakelite
2. Urea-formaldehyde resin
3. PVC
4. Melamine-formaldehyde resin
Polyacrylonitrile, characterized by the 
1.
2.
3.
4.
True statements regarding polymers are:
a. Polymers have a low molecular weight.
b. Polymers have high viscosity.
c. Polymers scatter light.
d. Polymers do not carry any charge.
1. a, b and c
2. a, b and d
3. b, c and d
4. a, c and d
The polymer that can't be prepared by condensation polymerization is :
1. Dacron
2. Nylon-6
3. Glyptal
4. PTFE
Match the polymers given in Column-I with their repeating units given in Column-II. Choose the correct option from the codes given below :
| Column-I | Column-II |
| (a) Polystyrene | (p) 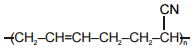 |
| (b) Novolac | (q) 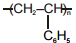 |
| (c) Buna-N | (r) 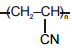 |
| (d) Acrilan | (s) 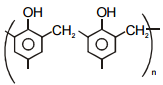 |
| (a) | (b) | (c) | (d) | |
| 1. | (s) | (r) | (q) | (p) |
| 2. | (r) | (p) | (s) | (q) |
| 3. | (p) | (q) | (r) | (s) |
| 4. | (q) | (s) | (p) | (r) |
Match the polymer of Column I with the correct monomer of Column II
| Column I | Column II |
| A. High-density polymer | 1. Isoprene |
| B. Neoprene | 2. Tetrafluoroethene |
| C. Natural rubber | 3. Chloroprene |
| D. Teflon | 4. Ethene |
Codes
| A | B | C | D | |
| 1. | 2 | 3 | 4 | 1 |
| 2. | 3 | 1 | 4 | 2 |
| 3. | 4 | 3 | 1 | 2 |
| 4. | 4 | 3 | 2 | 1 |






