The aromatic structure(s) out of given structures is/are-
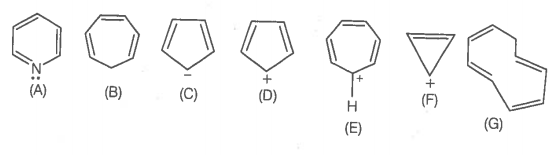
| 1. | A, C, D, F & G only | 2. | A & D only |
| 3. | A, C, E, F only | 4. | All are aromatic |
Subtopic: Aromaticity & Polarity |
81%
Level 1: 80%+
Hints
Links
The aromatic compound among the following is:-
| 1. | 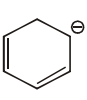 |
2. |  |
| 3. | 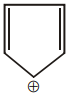 |
4. | 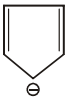 |
Subtopic: Aromaticity & Polarity |
77%
Level 2: 60%+
Hints
Links
The hydrocarbons having the lowest dipole moment among the following is:
1.
2. CH3 - C ≡ C - CH3
3. CH3 CH2 CH = CH2
4. CH2 = CH - C ≡ CH
Subtopic: Aromaticity & Polarity |
70%
Level 2: 60%+
Hints
The Huckel's rule-based aromaticity is shown by:
| (A) |  |
(B) |  |
| (C) |  |
(D) |  |
| (E) |  |
(F) |  |
| 1. | A, B, D only | 2. | B, D only |
| 3. | B, D, E and F only | 4. | A, B, D, E & F only |
Subtopic: Aromaticity & Polarity |
Level 3: 35%-60%
Hints
Links



