Select Chapter Topics:
Among the given reactions, which one does not result in the formation of a primary amine as the product?
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Amines - Preparation & Properties |
76%
Level 2: 60%+
NEET - 2023
Hints
Identify the product in the following reaction:


| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Diazonium Salts: Preparation, Properties & Uses |
57%
Level 3: 35%-60%
NEET - 2023
Hints
The least basic compounds/species among the following is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Amines - Preparation & Properties |
Level 3: 35%-60%
NEET - 2023
Hints
Which of the following is the correct sequence of reagents for converting 4-nitrotoluene to 2-bromotoluene?
| 1. | NaNO2/HCl; Sn/HCl; Br2; H2O/H3PO2 |
| 2. | Sn/HCl; NaNO2/HCl; Br2; H2O/H3PO2 |
| 3. | Br2; Sn/HCl; NaNO2/HCl; H2O/H3PO2 |
| 4. | Sn/HCl; Br2; NaNO2/HCl; H2O/H3PO2 |
Subtopic: Diazonium Salts: Preparation, Properties & Uses |
Level 3: 35%-60%
NEET - 2023
Hints
Given below are two statements:
In the light of the above statements, choose the most appropriate answer from the options given below:
| Statement I: | Primary aliphatic amines react with HNO2 to give unstable diazonium salts. |
| Statement II | Primary aromatic amines react with HNO2 to form diazonium salts which are stable even above 300 K. |
| 1. | Statement I is incorrect but Statement II is correct |
| 2. | Both Statement I and II are correct |
| 3. | Both Statement I and II are incorrect |
| 4. | Statement I is correct but Statement II is incorrect |
Subtopic: Diazonium Salts: Preparation, Properties & Uses |
53%
Level 3: 35%-60%
NEET - 2022
Hints
The product formed from the following reaction sequence is
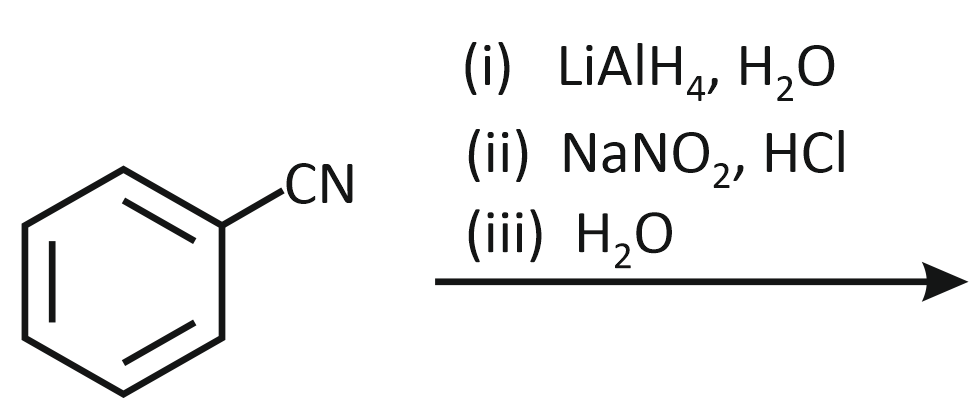
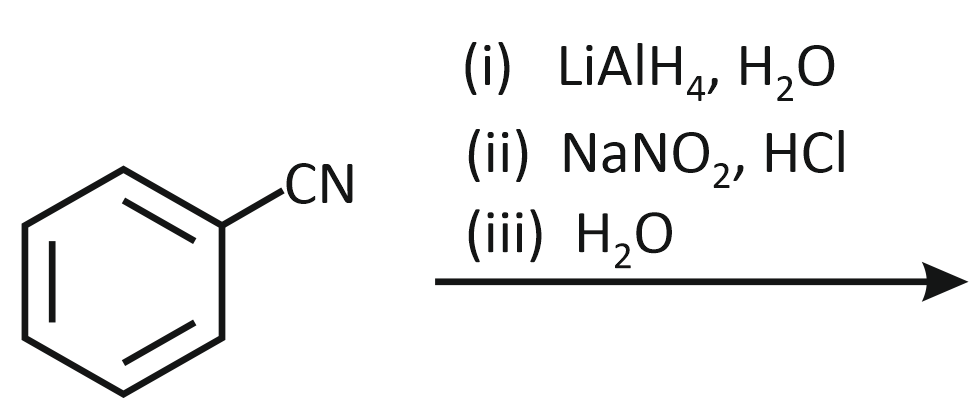
| 1. | 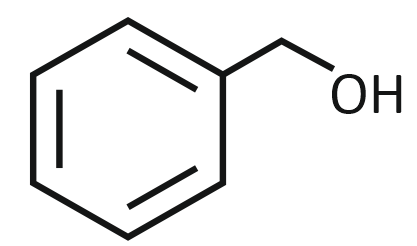 |
2. | 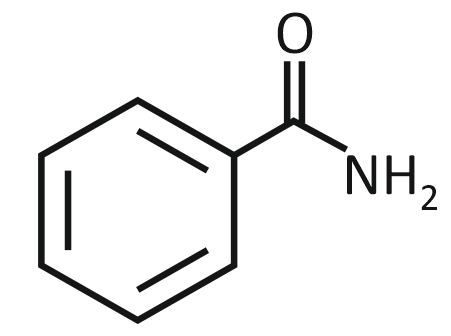 |
| 3. | 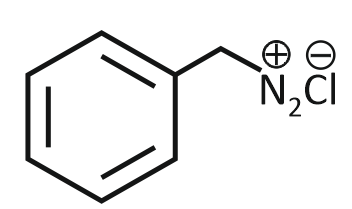 |
4. | 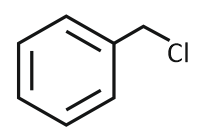 |
Subtopic: Diazonium Salts: Preparation, Properties & Uses | Mechanism |
75%
Level 2: 60%+
NEET - 2022
Hints
Match list-I with list-II:
| List-I (Amines) | List-II (pKb values) | ||
| (a) | N-Methylmethanamine | (i) | 9.30 |
| (b) | Ammonia | (ii) | 9.38 |
| (c) | N-Methylaniline | (iii) | 4.75 |
| (d) | Benzenamine | (iv) | 3.27 |
| (a) | (b) | (c) | (d) | |
| 1. | (iv) | (ii) | (i) | (iii) |
| 2. | (iv) | (iii) | (i) | (ii) |
| 3. | (iii) | (iv) | (i) | (ii) |
| 4. | (i) | (iv) | (iii) | (ii) |
Subtopic: Amines - Preparation & Properties |
70%
Level 2: 60%+
NEET - 2022
Hints
The major product (P) formed in the following reaction sequence is :
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Amines - Preparation & Properties |
69%
Level 2: 60%+
NEET - 2022
Hints
Match Column-I (Reaction) with Column-II (Product formed) and mark the appropriate choice:
| Column-I (Reaction) |
Column-II (Product formed) |
||
| (a) | Gabriel synthesis | (i) | Benzaldehyde |
| (b) | Kolbe synthesis | (ii) | Ethers |
| (c) | Williamson synthesis | (iii) | Primary amines |
| (d) | Etard reaction | (iv) | Salicylic acid |
| (a) | (b) | (c) | (d) | |
| 1. | (iii) | (i) | (ii) | (iv) |
| 2. | (ii) | (iii) | (i) | (iv) |
| 3. | (iv) | (iii) | (i) | (ii) |
| 4. | (iii) | (iv) | (ii) | (i) |
Subtopic: Amines - Preparation & Properties |
82%
Level 1: 80%+
NEET - 2022
Hints
Identify the compound that will react with Hinsberg's reagent to give a solid which dissolves in alkali.
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Identification of Primary, Secondary & Tertiary Amines |
81%
Level 1: 80%+
NEET - 2021
Hints







