The correct statement regarding the comparison of staggered and eclipsed conformations of ethane is:
| 1. | The eclipsed conformation of ethane is more stable than staggered conformation because eclipsed conformation has no torsional strain. |
| 2. | The eclipsed conformation of ethane is more stable than staggered conformation even though the eclipsed conformation has a torsional strain. |
| 3. | The staggered conformation of ethane is more stable than eclipsed conformation because staggered conformation has no torsional strain. |
| 4. | The staggered conformation of ethane is less stable than eclipsed conformation because staggered conformation has a torsional strain. |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The most suitable reagent for the following conversion is-
| 1. | Hg2+/ H+, H2O | 2. | Na/liquid NH3 |
| 3. | H2, Pd/C, quinoline | 4. | Zn/HCl |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Product \(Z\) in the above-mentioned reaction is:
1. \(\mathrm{CH}_3-\left(\mathrm{CH}_2\right)_3-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
2. \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3\)
3. \(\mathrm{CH}_3\left(\mathrm{CH}_2\right)_4-\mathrm{O}-\mathrm{CH}_3\)
4. \(\mathrm{CH}_3 \mathrm{CH}_2-\mathrm{CH}\left(\mathrm{CH}_3\right)-\mathrm{O}-\mathrm{CH}_2 \mathrm{CH}_3 \)

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The incorrect IUPAC name among the following is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Which compound among the following has the highest boiling point?
| 1. | Iso-octane | 2. | n-Octane |
| 3. | 2,2,3,3-Tetramethyl butane | 4. | n-Butane |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Which of the following hydrocarbon fuels has the highest octane rating?
1. Methane
2. Ethane
3. Iso-octane
4. Triptane
undergoes Wurtz reaction to give-
| 1. | Propane + Ethane | 2. | Propane |
| 3. | Propane + Ethane + Butane | 4. | Propane + Butane |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
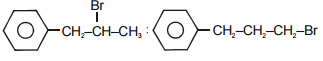
The main product A and B in the above mentioned reaction are respectively-
1.
2.
3. 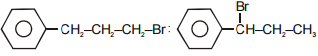
4. 

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
3-Hexyne can be converted to trans-3-Hexene by the action of:
1. - Pd/
2. Li-Liq.
3. - Pt
4.

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The major product in the above-mentioned reaction is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.











