A compound having the shortest carbon-carbon bond length among the following is :
1. Benzene
2. Ethene
3. Ethyne
4. Ethane

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The most suitable reagent among the following that is used to distinguish compound (III) from the rest of the compounds is -
I.
II.
III.
IV.
| 1. | Br2/CCl4 | 2. | Br2/CH3COOH |
| 3. | Alk.KMnO4 | 4. | Ammoniacal AgNO3 |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The reaction,
+ + HCl
is an example of:
1. Friedel-Crafts reaction
2. Kolbe's synthesis
3. Wurtz's reaction
4. Grignard synthesis

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
2-Methylpropene and But-1-ene can be distinguished by :
1. Baeyer's reagent
2. Ammoniacal
3. solution
4. ,

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Xylene, on oxidation with acidic , gives:
| 1. | Phthalic acid | 2. | Isophthalic acid |
| 3. | Terephthalic acid | 4. | All of the above |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
RCOCl and are used in the Friedel-Crafts reaction.
The electrophile among the following is:
1.
2. RCOCl
3. O
4.

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
3-Hexyne can be converted to trans-3-Hexene by the action of:
1. - Pd/
2. Li-Liq.
3. - Pt
4.

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Nitrobenzene on reaction with conc. HNO3/H2SO4 at 80-100C forms -
1. 1,2-Dinitrobenzene
2. 1,3-Dinitrobenzene
3. 1,4-Dinitrobenzene
4. 1,2,4-Trinitrobenzene

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The D in the above-mentioned reaction is -
| 1. | 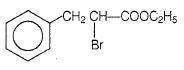 |
2. | 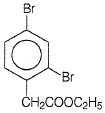 |
| 3. | 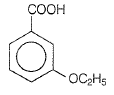 |
4. | 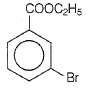 |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The reactants used in Friedel-Craft's alkylation are:
1. C6H6 + NH2
2. C6H6 + CH4
3. C6H6 + CH3Cl
4. C6H6 + CH3COCl

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.



