The major product obtained in the given reaction is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The mechanism & intermediate involved in the above reaction are:
1. Aromatic electrophilic substitution & carbocation
2. Aromatic Nucleophilic substitution & carbanion
3. Aromatic free radical substitution & Free radical
4. Carbene based substitution reaction & Carbene
A compound among the following that does not undergo Friedel-Craft's reaction easily is-
1. Cumene
2. Xylene
3. Nitrobenzene
4. Toluene

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The compounds among the following that can be generated by Friedel craft acylation is/are -
| I. |  |
| II. |  |
| III. |  |
| IV. |  |
| 1. | II, III and IV | 2. | I, III and IV |
| 3. | I and II | 4. | II and III |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Cyclopentadiene is much more acidic than cyclopentane, beacuse -
1. Cyclopentadiene has conjugated double bonds.
2. Cyclopentadiene has both sp2 and sp3 hybridized carbon atoms.
3. Cyclopentadiene is a strain-free cyclic system.
4. Cyclopentadienyl anion ion, the conjugate base of cyclopentadiene, is an aromatic species and hence has higher stability.

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
A compound among the following that gives the maximum % of meta product on nitration
(using HNO3/H2SO4) is :
1. Toluene
2. Aniline
3. Benzene
4. Isopropyl benzene

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The major product in the following reaction is-
| 1. | 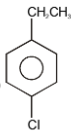 |
2. | 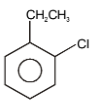 |
| 3. | 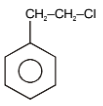 |
4. | 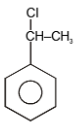 |
The D in the above-mentioned reaction is -
| 1. | 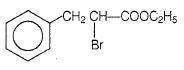 |
2. | 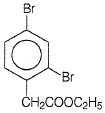 |
| 3. | 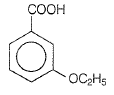 |
4. | 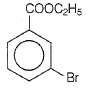 |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The product B in the above mentioned reaction is -
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Consider the following reaction:-
A and B are isomeric compounds. Additionally, A is a monochloro derivative. These two can be differentiated easily by using-
1. AgN(aq)
2.
3. Conc.HN/
4. NaCl(aq)

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.







