undergoes Wurtz reaction to give-
| 1. | Propane + Ethane | 2. | Propane |
| 3. | Propane + Ethane + Butane | 4. | Propane + Butane |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
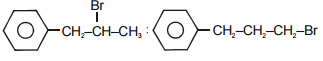
The main product A and B in the above mentioned reaction are respectively-
1.
2.
3. 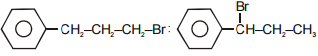
4. 

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
3-Hexyne can be converted to trans-3-Hexene by the action of:
1. - Pd/
2. Li-Liq.
3. - Pt
4.

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The major product in the above-mentioned reaction is:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.

The final product in the above sequence of reactions is-
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
A compound among the following that does not undergo Friedel-Craft's reaction easily is-
1. Cumene
2. Xylene
3. Nitrobenzene
4. Toluene

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.






