A non-aromatic compound among the following compounds is:
1.

2.

3.

4.






To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
A compound among the following that gives the maximum % of meta product on nitration
(using HNO3/H2SO4) is :
1. Toluene
2. Aniline
3. Benzene
4. Isopropyl benzene

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The action of AlCl3 in Friedel Craft's reaction is:
1. To absorb HCl
2. To release HCl
3. To produce an eleçtrophile
4. To produce nucleophile

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
n-Butylbenzene on oxidation with hot alkaline KMnO4 gives:
| 1. | Benzoic acid | 2. | Butanoic acid |
| 3. | Benzyl alcohol | 4. | Benzaldehyde |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The compound among the following that undergoes bromination of its aromatic ring
(electrophilic aromatic substitution) at the fastest rate is-
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Consider the following sequence of reactions.
The products (B) and (C) are respectively-
1. Benzaldehyde, and acetaldehyde
2. Benzoic acid, and acetic acid
3. Phenol, and propionaldehyde
4. Phenol, and acetone

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
In the above compound, Cl will liberate easily in the form of-
1.
2. Cl-
3. Cl
4. Cl2+

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
(A) C8H10 (B) C8H6O4 C8H5BrO4 (C) (one-product only)
The structure of A in the above-mentioned reaction is-
| 1. |  |
2. |  |
| 3. |  |
4. |  |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The product (B) in the above-mentioned reaction is-
| 1. | 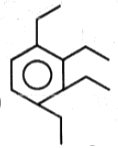 |
2. | 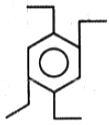 |
| 3. | 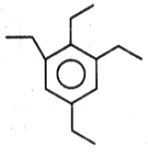 |
4. | 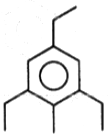 |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
Cyclopentadiene is much more acidic than cyclopentane, beacuse -
1. Cyclopentadiene has conjugated double bonds.
2. Cyclopentadiene has both sp2 and sp3 hybridized carbon atoms.
3. Cyclopentadiene is a strain-free cyclic system.
4. Cyclopentadienyl anion ion, the conjugate base of cyclopentadiene, is an aromatic species and hence has higher stability.

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.




