The correct hybridization states of carbon atoms in the following compound are -
| 1. | C1= sp , C2= sp3 , C3= sp2 | 2. | C1= sp2 , C2= sp3 , C 3 = sp3 |
| 3. | C1= sp2 , C2= sp2 , C 3 = sp | 4. | C1= sp3 , C2= sp3 , C 3 = sp3 |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The number of σ and π bonds in the molecule are -
| 1. | 6 C – C sigma ( σ C - C ) bonds, 5 C–H sigma ( ( σ C - H ) bonds, and 3 C=C pi ( π C - C ) |
| 2. | 6 C – C sigma ( σ C - C ) bonds, 5 C–H sigma ( ( σ C - H ) bonds, and 2 C=C pi ( π C - C ) |
| 3. | 6 C – C sigma ( σ C - C ) bonds, 6 C–H sigma ( ( σ C - H ) bonds, and 3 C=C pi ( π C - C ) |
| 4. | 6 C – C sigma ( σ C - C ) bonds, 6 C–H sigma ( ( σ C - H ) bonds, and 2 C=C pi ( π C - C ) |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The number of primary carbon atoms in the following compound are:
| 1. | 6 | 2. | 2 |
| 3. | 4 | 4. | 3 |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The \(C - H\) bond distance is longer in -
| 1. | \(C_2H_2\) | 2. | \(C_2H_4\) |
| 3. | \(C_2H_6\) | 4. | \(C_2H_2Br_2\) |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.

| 1. | 4 | 2. | 8 |
| 3. | 12 | 4. | 16 |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
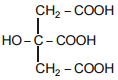
The IUPAC name of the above mentioned compound is -
1. Citric acid
2. 3-Hydroxy pentane-1,5-dioic acid
3. 2-Hydroxypropane-1,2,3-tricarboxylic acid
4. 2-Carboxy-2-hydroxy propane-1,3-dicarboxylic acid

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
1. 2–Bromo-3–methylbutanoic acid
2. 2-Methyl-3-bromobutanoic acid
3. 3-Bromo-2-methylbutanoic acid
4. 3-Bromo-2,3-dimethylpropanoic acid.

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
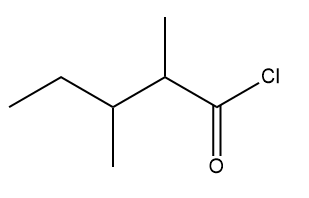
The IUPAC name of the above mentioned compound is -
1. 3, 4-Dimethylpentanoyl chloride
2. 1-Chloro-1-oxo-2,3-dimethylpentane
3. 2-Ethyl-3-methylbutanoylchloride
4. 2, 3-Dimethylpentanoyl chloride

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.
The correct IUPAC name, among the following, is-
| 1. | Prop-3-yn-1-ol | 2. | But-4-ol-4-yne |
| 3. | But-3-ol-2-yne | 4. | But-3-yn-1-ol |

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.

The IUPAC name of the above-mentioned compound is:
1. Pent-1-en-3-yne
2. Pent-1-ene-4-yne
3. Pent-4-yn-1-ene
4. Pent-1-en-4-yne

To unlock all the explanations of this course, you need to be enrolled.

To unlock all the explanations of this course, you need to be enrolled.




