Select Chapter Topics:
For the given reaction:

'P' is:
1.

2.

3.

4.





Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
From NCERT
NEET - 2024
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Hints
To view explanation, please take trial in the course.
NEET 2026 - Target Batch - Vital
Which of the following statements are incorrect in relation to the halogenation of alkane?
1. a, c, d
2. a, b, c
3. c, d, b
4. None of the above
| a. | The reactivity of chlorine is less than that of bromine towards alkanes. |
| b. | For the photochemical chlorination of methane, Cl is formed in the slowest step. |
| c. | Free radicals are pyramidal intermediates, stabilized by hyperconjugation and resonance. |
| d. | Bromine has much higher regioselectivity than chlorine in abstracting 3° hydrogen. |
2. a, b, c
3. c, d, b
4. None of the above
Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Which of the following reactions gives a meso-compound as the main product?
1. a, b
2. b, c
3. c, d
4. None of the above
| a. |  |
| b. | 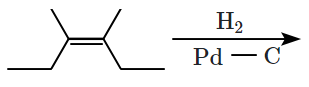 |
| c. |  |
| d. |  |
1. a, b
2. b, c
3. c, d
4. None of the above
Subtopic: Alkanes, Alkenes and Alkynes - Chemical Properties |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Why does the melting point of straight-chain hydrocarbons increase as the number of carbon atoms in the chain increases?
1. Due to the increasing mass of the compounds.
2. Due to the increasing polarity of the compounds.
3. Due to the increasing number of induced dipoles per molecule.
4. Due to the increased probability of hydrogen bonds.
1. Due to the increasing mass of the compounds.
2. Due to the increasing polarity of the compounds.
3. Due to the increasing number of induced dipoles per molecule.
4. Due to the increased probability of hydrogen bonds.
Subtopic: Aliphatic Hydrocarbon- Physical Properties |
Please attempt this question first.
Hints
Please attempt this question first.

The major product (F) of the above-given reaction sequence is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aromatic Hydrocarbons - Reactions & Mechanism |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Cyclohexene on ozonolysis by reaction with zinc dust and water gives compound E. Compound E on further treatment with aqueous \(KOH\) yields compound F. Compound F is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Aliphatic Hydrocarbon - Methods of Preparation |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Why does the addition of \(\text{HI}\) in the presence of a peroxide catalyst not follow anti-Markovnikov's rule?
| 1. | \(HI\) is a strong reducing agent. |
| 2. | The \(\text{H–I}\) bond is too strong to be broken homolytically. |
| 3. | The \(I\) atom combines with \(H\) atom to give back \(HI.\) |
| 4. | The iodine atom is not reactive enough to add across a double bond. |
Subtopic: Aliphatic Hydrocarbon - Methods of Preparation |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Find out the correct statement:
1. Only B is correct.
2. A and C.
3. B and C.
4. Only A is correct.
| A. | The number of possible theoretical conformations of ethane is 6. |
| B. | In the conformations of ethane, the staggered form has the least torsional strain, and the eclipsed form has the maximum torsional strain. |
| C. | Hückel Rule: The presence of (4n + 2) π electrons in the ring where n is an integer (n = 1, 2, ......). |
2. A and C.
3. B and C.
4. Only A is correct.
Subtopic: Aliphatic Hydrocarbon- Physical Properties | Aromatic Hydrocarbons - Nomenclature, Isomerism & Huckel's Rule |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Among the following, the compound which contains the fewest tertiary carbons is:
| 1. | \((CH_3)_3C(CH_2)_2CH_3~~~~~~~~~~~~~\) |
| 2. | 4-isobutyl heptane |
| 3. |  |
| 4. |  |
Subtopic: Aliphatic Hydrocarbon- Physical Properties |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Consider the following statements:
The correct statements is/are:
| a. | Benzene hexachloride is an aromatic compound. |
| b. | \(-NHCOCH_3\) is meta directing group towards aromatic electrophilic substitution reaction. |
| c. | In an arenium ion, one of the carbon is sp3 hybridised. |
| 1. | (a) and (b) only | 2. | (a) and (c) only |
| 3. | (c) only | 4. | (a), (b) and (c) |
Subtopic: Aromatic Hydrocarbons - Benzene - Structure, Preparation & Chemical Reactions |
From NCERT
Please attempt this question first.
Hints
Please attempt this question first.
Select Chapter Topics:




