The compound that possesses the most acidic hydrogen atom, among the following, is:
| 1. | \(\mathrm{H}_3 \mathrm{C}-\mathrm{C} \equiv \mathrm{C}-\mathrm{H}\) |
| 2. | 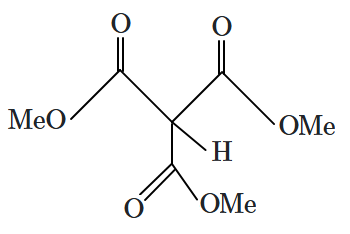 |
| 3. | 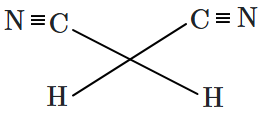 |
| 4. | 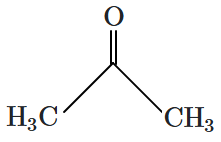 |
Subtopic: Acidic & Basic Character |
From NCERT
JEE
Please attempt this question first.
Hints
The basic strength of the following anions, ordered from most to least, is:
\(OH^- , HCOO^-, CH_3COO^- , \overline{O}R\\ (I)~~~~~~(II)~~~~~~~~~~~~(III)~~~~~~~~~~(IV)~~~~~~~~~~ \)
1. II > III > I > IV
2. IV > I > III > II
3. III > IV > I > II
4. I > IV > II > III
\(OH^- , HCOO^-, CH_3COO^- , \overline{O}R\\ (I)~~~~~~(II)~~~~~~~~~~~~(III)~~~~~~~~~~(IV)~~~~~~~~~~ \)
1. II > III > I > IV
2. IV > I > III > II
3. III > IV > I > II
4. I > IV > II > III
Subtopic: Acidic & Basic Character |
58%
From NCERT
JEE
Please attempt this question first.
Hints
Please attempt this question first.
