The Huckel's rule-based aromaticity is shown by:
(A)

(B)

(C)

(D)

(E)

(F)

1.
A, B, D only
2.
B, D only
3.
B, D, E and F only
4.
A, B, D, E & F only






The type of structural isomerism shown by given compounds is-
and 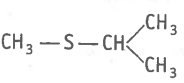
| 1. | Tautomerism | 2. | Positional isomerism |
| 3. | Functional isomerism | 4. | Ring Chain isomerism |
If a liquid compound decomposes at or below its boiling point, then the best method for purification is-
1. Distillation under reduced pressure
2. Azeotropic distillation
3. Gas chromatography
4. Sublimation
The most stable carbanion species among the following is-
| 1. | \(CCl^-_3\) | 2. | \(CH^-_3\) |
| 3. | \(CH_2Cl^-\) | 4. | \(CHCl^-_2\) |
The resonance hybrid structure will not exist for-
a. CH3OH
b. R - CONH2
c. CH3CH = CHCH2NH2
| 1. | a, and c | 2. | a, and b only |
| 3. | only a | 4. | b and c only |
Compare the stability of the two resonating structures given below and mark the correct option:
1. (I) is more stable than (II)
2. (II) is more stable than (I)
3. (I) and (II) both have the same stability
4. None of the above
| Assertion (A): | CCl4 doesn't give precipitate of AgCl on heating with AgNO3. |
| Reason (R): | CCl4 is a non-polar molecule. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
0.26 g of an organic compound gave 0.039 g of water and 0.245 g of carbon dioxide on combustion. The percentage of C in the organic compound is-
1. 35%
2. 25%
3. 2%
4. 90%
In an estimation of sulphur by the carius method, 0.2175 g of the substance gave 0.5825 g of BaSO4 . The percentage composition of S in the compound is-
1. 66%
2. 20%
3. 37%
4.82%
0.284 g of an organic substance gave 0.287 g AgCl in a carius method for the estimation of halogen. The percentage of Cl in the compound is-
1. 5%
2. 18%
3. 25%
4. 33%







