Value of for (strong electrolyte) in water at from the data below is:
| Conc. (mol/litre) | 0.25 | 1 |
| 260 | 250 |
1. 270 Ω-1 cm2 mol-1
2. 260 Ω-1 cm2 mol-1
3. 250 Ω-1 cm2 mol-1
4. 255 Ω-1 cm2 mol-1
During electrolysis of conc. H2SO4, perdisulphuric acid (H2S2O8), and O2 form in equimolar amount. The amount of H2 that will form simultaneously will be :
1. Thrice that of O2 in moles.
2. Twice that of O2 in moles.
3. Equal to that of O2 in moles.
4. Half of that of O2 in moles.
Consider the following Galvanic cell.
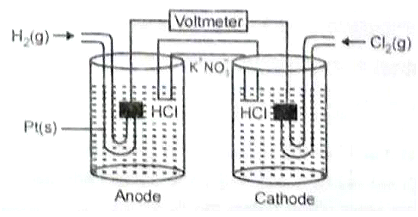
By what value the cell voltage change when concentration of ions in anodic and cathodic compartments both increased by a factor of 10 at 298
1. +0.0591
2. -0.0591
3. -0.1182
4. 0
Which characteristic of hydrogen makes it hazardous as a fuel?
1. High ignition with low combustion energy
2. High ignition with high combustion energy
3. Low ignition with low combustion energy
4. Low ignition with high combustion energy
The decreasing order of electrical conductivity of the following aqueous solutions is :
0.1 M Formic acid (A),
0.1 M Acetic acid (B),
0.1 M Benzoic acid (C)
1. A > B > C
2. A > C > B
3. C > B > A
4. C > A > B
Given below are the half-cell reactions :
The Eº for 3Mn2+ → Mn + 2Mn3+ will be :
1. – 2.69 V; the reaction will occur
2. – 0.33 V; the reaction will not occur
3. – 0.33 V; the reaction will occur
4. – 2.69 V; the reaction will not occur
The reduction potential of hydrogen half-cell will be negative if:
1. P(H2) = 1atm and [H+] = 2.0 M
2. P(H2) = 1 atm and [H+] = 1.0 M
3. P(H2) = 2 atm and [H+] = 1.0 M
4. P(H2) = 2 atm and [H+] = 2.0 M
For the following cell with hydrogen electrodes at two different pressures p1 and p2
Pt(H2) | H+(aq) |Pt (H2)
p1 1M p2
emf is given by:
1. \(\frac{R T}{F} \log _{e} \frac{P_{1}}{p_{2}}\)
2. \(\frac{R T}{2F} \log _{e} \frac{P_{1}}{p_{2}}\)
3. \(\frac{R T}{F} \log _{e} \frac{P_{2}}{p_{1}}\)
4. \(\frac{R T}{2F} \log _{e} \frac{P_{2}}{p_{1}}\)
| 1. | \(2.5 \times 10^{-3}\) | 2. | \(2 \times 10^{-3}\) |
| 3. | \(2.5 \times 10^{-4}\) | 4. | \(2 \times 10^{-4}\) |
1. \(2KCl + Br_2 \rightarrow 2KBr + Cl_2\)
2. \(2KF + I_2 \rightarrow 2KI + F_2\)
3. \(2 KClO_3 + I_2 \rightarrow 2KIO_3 + Cl_2\)
4. \(2KIO_3 + Cl_2 \rightarrow 2KClO_3 + I_2\)




