Which of the following compounds is/are aromatic alcohol?

1.
(A), (B), (C), (D)
2.
(A), (D)
3.
(B), (C)
4.
(A) Only

The proposed mechanism for hydration of ethene to yield ethanol is as follows:

The wrong step in the above mechanism is:
| 1. | Step 1 | 2. | Step 2 |
| 3. | Step 3 | 4. | Both steps 2 and 3 |
The IUPAC name of the product of o-hydroxy benzyl alcohol and PCl3 among the following is :
1. o-Hydroxy benzyl chloride
2. o-Chloromethylphenol
3. o-Chloromethylchlorobenzene
4. p-Hydroxymethylphenol
The major product (P) of the following reaction is:

| 1. |  |
2. |  |
| 3. |  |
4. |  |
What is the major product formed in the following reaction?

| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Select the correct option based on statements below:
| Assertion (A): | Phenol does not react with NaHCO3 but 2,4,6-trinitrophenol does react with the evolution of CO2 |
| Reason (R): | 2,4,6-trinitrophenol is a stronger acid than carbolic acid. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
1. CH3OH >C2H5OH >H2O
2. H2O >CH3OH > C2H5OH
3. CH3OH > H2O > C2H5OH
4. C2H5OH > CH3OH >H2O
The order of reactivity of the following Compounds in electrophilic monochlorination at the most favourable position is
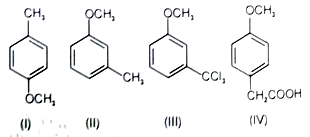
1. I < II < IV < III
2. III < IV < I < II
3. IV < III < II < I
4. III < II < IV < I
1-Phenoxypropane is treated with an excess of conc. Hl at 0C and the mixture of products is treated with thionyl chloride. The products formed are:
| 1. | n-propanol + Chlorobenzene |
| 2. | Phenol + n-propyl iodide |
| 3. | n-propyl chloride + Chlorobenzene |
| 4. | n-propyl chloride + Phenol |

1. t-Butylether
2. 2-Methylpropene
3. 2-Methylpent-1-ene
4. 2,2,3,3-Tetramethylbutane




