Select Chapter Topics:
Consider the following species:

What is the hybridization of the carbon carrying a negative charge in structures (I) and (II), respectively?

What is the hybridization of the carbon carrying a negative charge in structures (I) and (II), respectively?
| 1. | sp2 and sp2 | 2. | sp2 and sp3 |
| 3. | sp and sp2 | 4. | sp3 and sp3 |
Subtopic: Hybridisation & Structure of Carbon Compounds |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
The total number of substituents present in the following compound is:


| 1. | Four(4) | 2. | Five(5) |
| 3. | Seven(7) | 4. | Three(3) |
Subtopic: Nomenclature |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
What is correct about the following structure?

1. Total stereoisomers =4
2. Number of chiral carbons =1
3. Number of optical isomers =2
4. Number of meso compounds= 2

1. Total stereoisomers =4
2. Number of chiral carbons =1
3. Number of optical isomers =2
4. Number of meso compounds= 2
Subtopic: Stereo Isomers |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
How many geometrical isomers are possible for a compound with the molecular formula \(\mathrm{C_2BrClFI}\)?
1. 3
2. 4
3. 5
4. 6
1. 3
2. 4
3. 5
4. 6
Subtopic: Stereo Isomers |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Consider the following statements
The correct statements are:
| a. | In crystallization, an impure compound is dissolved in a solvent in which it is highly soluble at room temperature. |
| b. | In the positive resonance effect(+R), the transfer of electrons is always from an atom or substituted group attached to the conjugated system. |
| c. | One of the technological applications of fractional distillation is to separate different fractions of crude oil in the petroleum industry. |
| 1. | (a) and (b) only | 2. | (b) and (c) only |
| 3. | (a) and (c) only | 4. | (a), (b), and (c) |
Subtopic: Electron Displacement Effects |
52%
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
The correct arrangement of the following carbocations in decreasing order of their stability is:

1. A > B > C > D
2. B > C > A > D
3. C > B > A > D
4. C > A > B > D
Subtopic: Electron Displacement Effects |
57%
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
In which of the following, hyperconjugation effect is not observed?
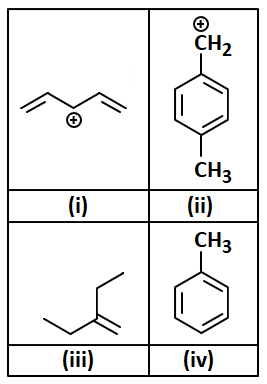
1. (i) and (ii)
2. (i), (ii) and (iii)
3. (i) only
4. (i), (ii) and (iv)

1. (i) and (ii)
2. (i), (ii) and (iii)
3. (i) only
4. (i), (ii) and (iv)
Subtopic: Electron Displacement Effects |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
Consider the resonance structure of CH3COOCH3 as follows:
The correct statement among the following is:
| 1. | I and II cannot be the major contributors to the real structure of CH3COOCH3 |
| 2. | I and II can be the major contributors to the real structure of CH3COOCH3 |
| 3. | I is more stable than II. |
| 4. | None of the above |
Subtopic: Electron Displacement Effects |
Level 4: Below 35%
Hints

In the above compound, at which bond proton attack fastest?
1. A
2. B
3. C
4. D
Subtopic: Acidic & Basic Character |
Level 3: 35%-60%
Hints
What is the correct order of stability for the following carbanions?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
1. (1) > (2) > (3) > (4)
2. (2) > (3) > (4) > (1)
3. (4) > (2) > (3) > (1)
4. (1) > (3) > (2) > (4)
Subtopic: Reaction Intermediates ; Preparation & Properties |
Level 3: 35%-60%
AIPMT - 2008
Hints
Select Chapter Topics:







