Select Chapter Topics:
Arrange the following amines in decreasing order of basicity:
1. (A) > (C) > (D) > (B)
2. (C) > (A) > (B) > (D)
3. (B) > (C) > (D) > (A)
4. (C) > (B) > (A) > (D)
| (A) |  |
(B) |  |
| (C) |  |
(D) |  |
2. (C) > (A) > (B) > (D)
3. (B) > (C) > (D) > (A)
4. (C) > (B) > (A) > (D)
Subtopic: Amines - Preparation & Properties |
52%
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Consider the given two statements:
| Statement I: | In the Hofmann rearrangement reaction, the alkyl group shifts from a carbonyl carbon atom to the nitrogen atom. |
| Statement II: | The tertiary amine formed in Hofman rearrangement reaction contains one carbon less than that present in the original amide. |
| 1. | Both Statement I & Statement II are true. |
| 2. | Both Statement I & Statement II are false. |
| 3. | Statement I is true while Statement II is false. |
| 4. | Statement I is false while Statement II is true. |
Subtopic: Amines - Preparation & Properties |
51%
Level 3: 35%-60%
JEE
Please attempt this question first.
Hints
Please attempt this question first.
| Assertion (A): | In strongly acidic solutions, aniline becomes more reactive towards electrophilic reagents. |
| Reason (R): | The amino group being completely protonated in a strongly acidic solution, the lone pair of electrons on the nitrogen is no longer available for resonance |
| 1. | Both (A) & (R) are True and the reason is the correct explanation of the (A). |
| 2. | Both (A) & (R) are True but the reason is not the correct explanation of the (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |
Subtopic: Amines - Preparation & Properties |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Match the organic compounds given in List–I with their corresponding pKb given in List–II:
| Amine (List–I) | List - II pKb (aqueous medium) | ||
| A. | Aniline | (i) | 9.0 |
| B. | Ethanamine | (ii) | 3.29 |
| C. | N-Ethylethanamine | (iii) | 3.25 |
| D. | N,N-diethylethanamine | (iv) | 3.0 |
| A | B | C | D | |
| 1. | (i) | (ii) | (iv) | (iii) |
| 2. | (i) | (iv) | (iii) | (ii) |
| 3. | (i) | (ii) | (iii) | (iv) |
| 4. | (ii) | (iii) | (iv) | (i) |
Subtopic: Amines - Preparation & Properties |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
The most reactive amine towards dilute hydrochloric acid is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Subtopic: Amines - Preparation & Properties |
Level 3: 35%-60%
Hints
Which of the following will produce the highest yield in Friedel Crafts reaction?
| 1. | 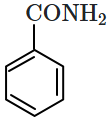 |
2. |  |
| 3. |  |
4. |  |
Subtopic: Amines - Preparation & Properties |
Level 3: 35%-60%
Please attempt this question first.
Hints
Please attempt this question first.
Consider the given two statements:
Options:
| Assertion (A): | p-Toluidine is a stronger base than m-toluidine. |
| Reason (R): | The methyl group from m-position exerts a smaller electron-donating inductive (+l) effect than from p-position. |
| 1. | Both (A) and (R) are True and (R) is the correct explanation for (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation for (A). |
| 3. | (A) is True but (R) is False. |
| 4. | Both (A) and (R) are False. |
Subtopic: Amines - Preparation & Properties |
Level 3: 35%-60%
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
Which of the following should be most volatile?
| I. | \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{NH}_2\) |
| II. | \(\left(\mathrm{CH}_3\right)_3 \mathrm{~N}\) |
| III. |  |
| IV. | \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_3\) |
| 1. | II | 2. | IV |
| 3. | I | 4. | III |
Subtopic: Amines - Preparation & Properties |
51%
Level 3: 35%-60%
Hints
In the following reaction sequence:

the major products X and Z are:

the major products X and Z are:
| 1. |  |
| 2. |  |
| 3. |  |
| 4. |  |
Subtopic: Amines - Preparation & Properties | Mechanism |
Level 4: Below 35%
Please attempt this question first.
Hints
Please attempt this question first.
\(R-COOH\xrightarrow[(2)~Br_2/KOH]{(1)~ND_3,~\Delta}A~\text(major).\)
The compound \('A'\) is:
1. \(R-COND_2\)
2. \(R-NH_2\)
3. \(R-ND_2\)
4. \(R-CONH_2\)
The compound \('A'\) is:
1. \(R-COND_2\)
2. \(R-NH_2\)
3. \(R-ND_2\)
4. \(R-CONH_2\)
Subtopic: Amines - Preparation & Properties |
Level 4: Below 35%
Please attempt this question first.
Hints
Please attempt this question first.






