The correct order of the leaving group ability is:
1.
\(\text{ OCOC}_2\text{H}_5~\)>\(\text{OC}_2\text{H}_5\) >\(\text {OSO}_2\text{Me}\) > \(\text {OSO}_2\text{CF}_3\)
2.
\(\text{OC}_2\text{H}_5\) > \(\text{ OCOC}_2\text{H}_5~\)> \(\text {OSO}_2\text{CF}_3\) > \(\text {OSO}_2\text{Me}\)
3.
\(\text {OSO}_2\text{CF}_3\) > \(\text {OSO}_2\text{Me}\) > \(\text{ OCOC}_2\text{H}_5~\) > \(\text{OC}_2\text{H}_5\)
4.
\(\text{ OCOC}_2\text{H}_5~\)>\(\text {OSO}_2\text{CF}_3\) > \(\text{OC}_2\text{H}_5\) > \(\text {OSO}_2\text{Me}\)
Consider the following resonating structures of HCOOH
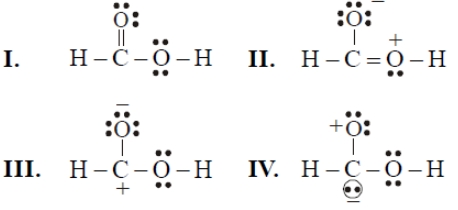
The order of stability is-
| 1. | I>II>III>IV | 2. | IV>I>II>III |
| 3. | I>III>II>IV | 4. | II>I>III>IV |
The number of chain isomers for and are, respectively :
1. 3,3
2. 3,5
3. 4,4
4. 3,4
Silver sulphate solution is used to separate:
1. Nitrate and bromide
2. Nitrate and chlorate
3. Bromide and iodide
4. Nitrate and nitrite
A compound that does not give a positive test in Lassaigne’s test for nitrogen is-
| 1. | Urea | 2. | Hydrazine |
| 3. | Azobenzene | 4. | Phenyl hydrazine |
The pair that represents chain isomers is-
| 1. | CH3CHCl2 and ClCH2CH2Cl | 2. | Propyl alcohol and Isopropyl alcohol |
| 3. | 2-Methylbutane and Neopentane | 4. | Diethyl ether and Dipropyl ether |
Fischer projection indicates:
1. Horizontal substituents above the plane.
2. Vertical substituents above the plane.
3. Both horizontal and vertical substituents below the plane.
4. Both horizontal and vertical substituents above the plane.
In an SN1 reaction on chiral center, there is:
1. 100% racemization
2. Inversion is more than retention leading to partial racemization.
3. 100% retention
4. 100% inversion
The general molecular formula, that represents the homologous serious of alkanols is:
1. \(C_{n} H_{2 n} O_{2}\)
2. \(C_{n} H_{2 n} O\)
3. \(C_{n} H_{2 n + 1} O\)
4. \(C_{n} H_{2 n + 2} O\)
1. E1
2. E2
3.
4. elimination






