Which of the following statement in the case of a thermodynamic process is not correct?
(The symbols carry their usual meaning)
1.
\(\Delta E_{\text{int}}=W;\) indicates an adiabatic process
2.
\(\Delta E_{\text{int}}=Q;\) suggests an isochoric process
3.
\(\Delta E_{\text{int}}=0;\) is true for a cyclic process
4.
\(\Delta E_{\text{int}}=-W;\) indicates an adiabatic processes
(The symbols carry their usual meaning)
1 kg of gas does 20 kJ of work and receives 16 kJ of heat when it is expanded between two states. The second kind of expansion can be found between the same initial and final states, which requires a heat input of 9 kJ. The work done by the gas in the second expansion will be:
| 1. | 32 kJ | 2. | 5 kJ |
| 3. | -4 kJ | 4. | 13 kJ |
A system is taken from state A to state B along two different paths, 1 and 2. If the heat absorbed and work done by the system along these two paths are respectively, then:
| 1. | \(Q_1=Q_2\) |
| 2. | \(W_1=W_2\) |
| 3. | \(Q_1-W_1=Q_2-W_2\) |
| 4. | \(Q_1+W_1=Q_2+W_2\) |
The figure below shows two paths that may be taken by a gas to go from state A to state C. In process AB, \(400~\text{J}\) of heat is added to the system and in process BC, \(100~\text{J}\) of heat is added to the system. The heat absorbed by the system in the process AC will be:
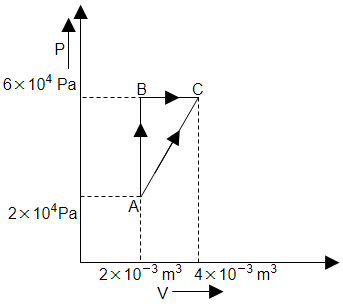
| 1. | \(380~\text{J}\) | 2. | \(500~\text{J}\) |
| 3. | \(460~\text{J}\) | 4. | \(300~\text{J}\) |
If ΔQ and ΔW represent the heat supplied to the system and
the work done on the system, respectively, then the first law of thermodynamics can be written as: (where ΔU is the internal energy)
1. ΔQ = ΔU + ΔW
2. ΔQ = ΔU – ΔW
3. ΔQ = ΔW – ΔU
4. ΔQ = –ΔU – ΔW
Can two isothermal curves cut each other?
| 1. | Never |
| 2. | Yes |
| 3. | They will cut when the temperature is 0°C. |
| 4. | Yes, when the pressure is equal to the critical pressure. |
The latent heat of vaporisation of water is \(2240~\text{J/gm}\). If the work done in the process of expansion of \(1~\text{g}\) is \(168~\text{J}\),
then the increase in internal energy is:
1. \(2408~\text{J}\)
2. \(2240~\text{J}\)
3. \(2072~\text{J}\)
4. \(1904~\text{J}\)
An ideal gas at \(27^{\circ}\mathrm{C}\) is compressed adiabatically to of its original volume. If , then the rise in temperature will be:
1. 450 K
2. 375 K
3. 225 K
4. 405 K
A polyatomic gas \(\left(\gamma = \frac{4}{3}\right)\) is compressed to \(\frac{1}{8}\) of its volume adiabatically. If its initial pressure is \(P_0,\) its new pressure will be:
| 1. | \(8P_0\) | 2. | \(16P_0\) |
| 3. | \(6P_0\) | 4. | \(2P_0\) |
A unit mass of a liquid with volume V1 is completely changed into a gas of volume V2 at a constant external pressure P and temperature T. If the latent heat of evaporation for the given mass is L, then the increase in the internal energy of the system is:
1. Zero
2.
3.
4. L


