A gas is taken through the cycle A→B→C→A, as shown. What is the net work done by the gas?

1. 2000J
2. 1000J
3. Zero
4. -2000J

1. 2000J
2. 1000J
3. Zero
4. -2000J
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio of CP/CV for the gas is equal to:
| 1. | 4/3 | 2. | 2 |
| 3. | 5/3 | 4. | 3/2 |
A thermodynamic system is taken through the cycle ABCD as shown in figure. Heat rejected by the gas during the cycle is
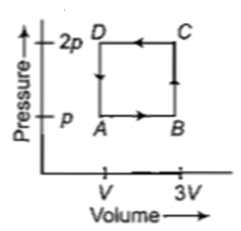
1. 2 pV
2. 4 pV
3.
4. pV
One mole of an ideal gas from an initial state A undergoes via two processes. It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P-V diagram representing the two processes is -
1. 
2. 
3. 
4. 
An ideal gas goes from state \(A\) to state \(B\) via three different processes, as indicated in the \(P\text-V\) diagram. If \(Q_1,Q_2,Q_3\) indicates the heat absorbed by the gas along the three processes and \(\Delta U_1, \Delta U_2, \Delta U_3\) indicates the change in internal energy along the three processes respectively, then:

| 1. | \({Q}_1>{Q}_2>{Q}_3 \) and \(\Delta {U}_1=\Delta {U}_2=\Delta {U}_3\) |
| 2. | \({Q}_3>{Q}_2>{Q}_1\) and \(\Delta {U}_1=\Delta {U}_2=\Delta {U}_3\) |
| 3. | \({Q}_1={Q}_2={Q}_3\) and \(\Delta {U}_1>\Delta {U}_2>\Delta {U}_3\) |
| 4. | \({Q}_3>{Q}_2>{Q}_1\) and \(\Delta {U}_1>\Delta {U}_2>\Delta {U}_3\) |
A mass of diatomic gas (=1.4) at a pressure of 2 atm is compressed adiabatically so that its temperature rise from to The pressure of the gas is final state is-
(1) 28 atm
(2) 68.7 atm
(3) 256 atm
(4) 8 atm
If represent the increase in internal energy and work done by the system respectively in a thermodynamical process,which of the following is true?
(1) in a adiabatic process
(2) in a isothermal process
(3) in adiabatic process
(4) in a isothermal process
A monoatomic gas at pressure and is compressed adiabatically to its original volume. What is the final pressure of the gas ?
1,
2.
3.
4.
The internal energy change in a system that has absorbed 2 kcal of heat and done 500 J of work is
1. 8900 J
2. 6400 J
3. 5400 J
4. 7900 J
In thermodynamic processes, which of the following statements is not true?
| 1. | In an adiabatic process, the system is insulated from the surroundings. |
| 2. | In an isochoric process, the pressure remains constant. |
| 3. | In an isothermal process, the temperature remains constant. |
| 4. | In an adiabatic process, \(P V^\gamma\) = constant. |






