Among the following ethers, the one that will produce methyl alcohol on treatment with hot concentrated HI is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
H2COH.CH2OH on heating with periodic acid gives :-
1. 2CO2
2. 2HCOOH
3. CHO-CHO
4.
Acetophenone when reacted with a base, C2H5ONa, yields a stable compound which has the structure
The reaction:
Which of the following compounds will be formed?
(1)
(2)
(3)
(4)
The major organic product in the reaction, CH3 — O — CH(CH3)2 + HI Product is :
(1) CH3OH+(CH3)2CHI
(2) ICH3OCH(CH3)2
(3) CH3O CI(CH3)2
(4) CH3I+(CH3)2CHOH
Ethylene oxide when treated with Grignard reagent yields :
(1) secondary alcohol
(2) tertiary alcohol
(3) cyclopropyl alcohol
(4) primary alcohol
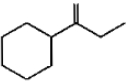
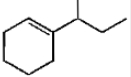
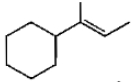
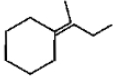
Which of the following is not the product of dehydration of
1. 
2. 
3. 
4. 
Reaction of phenol with chloroform in the presence of dilute sodium hydroxide finally
introduces, which one of the following functional group?
1. -CH2Cl
2. -COOH
3. -CHCl2
4. -CHO
The reaction
can be classified as
1. Alcohol formation reaction
2. Dehydration reaction
3. Williamson alcohol synthesis reaction
4. Williamson ether synthesis reaction
Which one is the most acidic compound ?







