For the indicator diagram given below, which of the following is not correct?

1.
Cycle II is a heat engine cycle.
2.
Net work is done on the gas in cycle I.
3.
Work done is positive for cycle I.
4.
Work done is positive for cycle II.

An ideal gas with adiabatic exponent y is heated at constant pressure and it absorbs Q heat. What fraction of this heat is used to perform external work?
1.
2.
3.
4.
Temperature is defined by
1. First Law of thermodynamics
2. Second Law of thermodynamics
3. Third Law of thermodynamics
4. Zeroth Law of thermodynamics
The P-V diagram for a thermodynamic system is shown in the figure. The work done by the system during the cyclic process ABCA is

1. Zero
2. 90 J
3. 30 J
4. 60 J
If is the work done in compressing an ideal gas from a given initial state through a certain volume isothermally and is the work done in compressing the same gas from the same initial state through the same volume adiabatically, then:
1.
2.
3.
4.
An ideal gas changes from state 'a' to state 'b' as shown in figure. What is the work done by the gas in the process?

1. zero
2. positive
3. negative
4. infinite
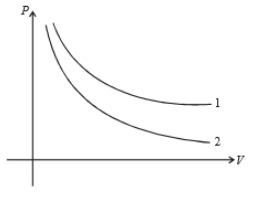

P-V graph of two gases during adiabatic processes are shown in figure. Plots 1 and 2 should correspond, respectively to :
1. He,
2. and He
3. He and Ar
4. and
For an ideal gas V-T curves at constant pressure and are shown in figure. From the figure


1.
2.
3.
4.
1. \(T\)
2. \(2T\)
3. \(\sqrt{2}T\)
4. \(\frac{T}{2}\)
A sink, that is, the system where heat is rejected, is essential for the conversion of heat into work. From which law does the above inference follow?
1. Zeroth
2. First
3. Second
4. Third






