A thermodynamic system is taken from an original state to an intermediate state by the linear process shown in the figure. Its volume is then reduced to the original value from \(\mathrm{E}\) to \(\mathrm{F}\) by an isobaric process. The total work done by the gas from \(\mathrm{D}\) to \(\mathrm{E}\) to \(\mathrm{F}\) is:

1. \(600 ~\text{J}\)
2. \(300 ~\text{J}\)
3. \(450 ~\text{J}\)
4. \(500 ~\text{J}\)

1. \(600 ~\text{J}\)
2. \(300 ~\text{J}\)
3. \(450 ~\text{J}\)
4. \(500 ~\text{J}\)
A refrigerator is to maintain eatables kept inside at \(9^\circ \text{C}.\) If room temperature is \(36^\circ \text{C},\) the coefficient of performance is:
1. \(9.3\)
2. \(12.4\)
3. \(11.2\)
4. \(10.4\)
In thermodynamics, the Zeroth law is related to:
1. Work done
2. Thermal equilibrium
3. Entropy
4. Diffusion
In the following figures (a) to (d), the variation of volume by change of pressure is shown. The gas is taken along the path ABCDA. The change in internal energy of the gas will be:
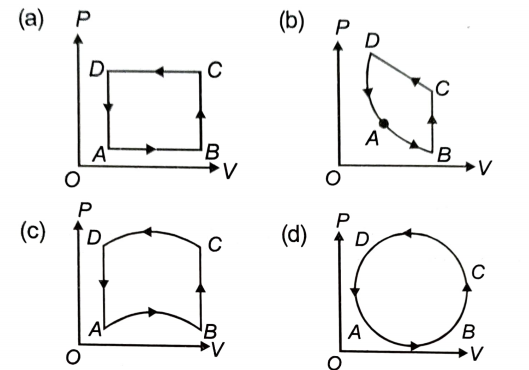
1. positive in all cases from (a) to (d).
2. positive in cases (a), (b) and (c) but zero in case (d).
3. negative in cases (a), (b) and (c) but zero in case (d).
4. zero in all four cases.
(where symbols have their usual meanings)
1. \(C_P = \frac{\gamma R}{\gamma-1 }\)
2. \(C_P-C_V= R\)
3. \(\Delta U = \frac{P_fV_f-P_iV_i}{1-\gamma}\)
4. \(C_V = \frac{R}{\gamma-1 }\)
Select the incorrect statement about the specific heats of a gaseous system.
1. Specific heat at no exchange condition,
2. Specific heat at a constant temperature,
3. Specific heat at constant pressure,
4. Specific heat at constant volume,
A certain amount of an ideal monatomic gas needs 20 J of heat energy to raise its temperature by at constant pressure. The heat needed for the same temperature rise at constant volume will be:
1. 30 J
2. 12 J
3. 200 J
4. 215.3 J
Work done in the cyclic process shown in the figure is:
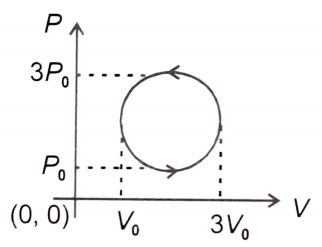
1.
2.
3.
4.
For a certain process, the pressure of a diatomic gas varies according to the relation , where a is a constant. What is the molar heat capacity of the gas for this process?
1.
2.
3.
4.






