My Notes
(CH3)3CCI + (CH3)3C K+ → Product
1. SN Product will be more
2. E2 Product will be more
3. Both will be the same
4. None of the above
Consider the following reaction:-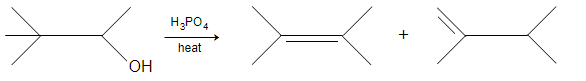
Which response contains all the correct statement about this process?
(1)Dehydration
(2)E2 mechanism
(3)Carbon skeleton migration
(4)Most stable alkene will form
(5)Single-step reaction
(a)1,3 (b)1,2,3 (c)1,2,5 (d)1,3,4
In E2 elimination, some compounds follow Hofmann's rule which means:
1. the double bond goes to the most substituted carbon
2. the compound is resistant to elimination
3. no double bond is formed
4. the double bond goes mainly towards the least substituted carbon
Consider the following statements :
(i) Pyrolytic elimination is always syn elimination.
(ii) In pyrolytic elimination, product formation takes place by most stable eclipsed conformation.
(iii) In pyrolytic elimination, product formation takes place by Hofmann rule.
(iv) In pyrrolytic elimination, product formation takes place by Saytzeff rule.
Of these statements :
(1) (i), (ii) and (iv) are correct
(2) (i), (ii) and (iii) are correct
(3) (i) and (ii) are correct
(4) (ii) and (iii) are correct
