Why should a magnesium ribbon be cleaned before burning in air?
Write the balanced equation for the following chemical reactions:
(i) Hydrogen + Chlorine Hydrogen chloride
(ii) Barium chloride + Aluminium sulphate Barium sulphate + Aluminium chloride
(iii) Sodium + Water Sodium hydroxide + Hydrogen
Write a balanced chemical equation with state symbols for the following reactions:
(i) Solutions of barium chloride and sodium sulphate in water react to give insoluble barium sulphate and the solution of sodium chloride.
(ii) Sodium hydroxide acid solution (in water) reacts with hydrochloric acid solution (in water) to produce sodium chloride solution and water.
A solution of a substance X is used for while washing.
(i) Name the substance X and write its formula.
(ii) Write the reaction of the substance X named in with water.
Why is the amount of gas collected in one of the test tubes in activity 7 double of the amount collected in the other? Name this gas.
Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Give an example of a double displacement reaction other than the reaction of barium chloride with sodium sulphate.
Identify the substances that are oxidised and the substances that are reduced in the following reactions.
Which of the statements about the reaction below are incorrect?
(i) Lead is getting reduced
(ii) Carbon dioxide is getting oxidised
(iii) Carbon is getting oxidised
(iv) Lead oxide is getting reduced
The above reaction is an example of a
1. combination reaction
2. double displacement reaction
3. decomposition reaction
4. displacement reaction
What happens when dil. HCl is added to iron filings? Tick the correct answer.
1. Hydrogen gas and iron chloride are produced
2. Chlorine gas and iron hydroxide are produced
3. No reaction takes place
4. Iron salt and water are produced
What is a balanced chemical equation? Why should chemical equations be balanced?
Translate the following statements into chemical equations and then balance them:
(i) Hydrogen gas combines with nitrogen to form ammonia.
(ii) Hydrogen sulphide gas burns in air to give water and sulphur dioxide.
(iii) Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.
(iv) Potassium metal reacts with water giving potassium hydroxide and hydrogen gas.
Balance the following chemical equations:
Write the balanced chemical equation for the following reactions:
(i) Calcium hydroxide+Carbon dioxide Calcium carbonate+Water
(ii) Zinc+Silver nitrate Zinc nitrate+Silver
(iii) Aluminium+Copper chloride Aluminium chloride+Copper
(iv) Barium chloride+Potassium sulphate Barium sulphate+Potassium chloride
Write the balanced chemical equation for the following and identify the type of reaction in each case.
What does one mean by exothermic and endothermic reactions? Give examples.
Why is respiration considered as an exothermic reaction? Explain.
Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Write one equation each for decomposition reactions where energy is supplied in the form of heat, light and electricity.
What is the difference between displacement and double displacement reaction? Write equations for these reactions.
In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
What do you mean by a precipitation reaction? Explain by giving examples.
Explain the following in terms of gain or loss of oxygen with two examples each.
(i) Oxidation (ii) Reduction
A shiny brown coloured element X on heating in the air becomes black in colour. Name the element X and the black coloured compound formed.
Why do we apply paint on iron articles?
Oil and fat containing food items are flushed with nitrogen. Why?
Explain the following terms with one example of each:
(i) Corrosion (ii) Rancidity
In the reaction,
write the values of X and Y.
Complete the missing component/variable given as X and Y in the following reaction.
The carbonate of metal X is a white solid. It decomposes when heated to form carbon dioxide and yellow solid oxide. What is metal X?
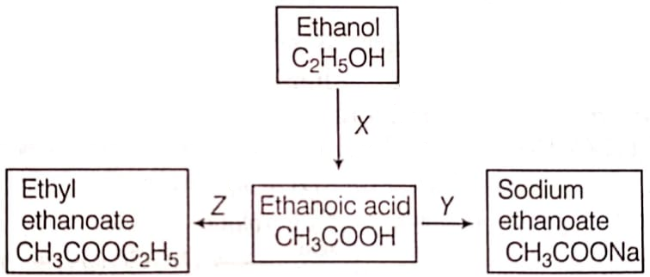
Write a balanced chemical equation for the following reaction. Ethanol is warmed with ethanoic acid to form ethyl acetate in the presence of conc. H2SO4.
Identify the reducing agent in the following reaction.
Identify the oxidising agent in the following:
Identify the type of reaction given below:
Double displacement reactions are also known as precipitation reactions. Why?
Why is photosynthesis considered an endothermic reaction?
What is the main reason behind various metals such as iron gets wasted every year in our country?
Write the difference between a physical change and a chemical change.
Using a suitable chemical equation, justify that some chemical reactions are determined by
(i) change in colour.
(ii) change in temperature.
Change the following reactions into balanced chemical equations.
(i) Manganese dioxide is heated with aluminium powder.
(ii) Iron is treated with steam.
Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
(i) In thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
(ii) Magnesium ribbon is burnt in an atmosphere of nitrogen gas to form solid magnesium nitride.
Write a balanced chemical equation for the following reactions.
(i) Silver bromide on exposure to sunlight decomposes into silver and bromine.
(ii) Sodium metal reacts with water to form sodium hydroxide and hydrogen gas.
A substance X, which is an oxide of a group 2 element is used intensively in the cement industry. This element is present in bones also. On treatment with water, it forms a solution which turns red litmus blue. Identify X and also write the chemical reactions involved.
Zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not. Explain, why?
A white salt of lead on heating decomposes to give brown fumes and a residue is left behind.
(i) Name the salt.
(ii) Write the equation for the decomposition reaction.
Why do we store silver chloride in dark coloured bottles?
A metal salt MX when exposed to light, split up to form metal M and a gas X2. Metal M is used in making ornaments whereas gas X2 is used in making bleaching powder. The salt MX is itself used in black and white photography.
(i) Identify metal M and gas X2.
(ii) Mention the type of chemical reaction involved when salt MX is exposed to light.
Why do fireflies glow at night?
Which reaction below oxidation reactions?
Identify the oxidising agents (oxidants) in the following reactions.
A brown substance X on heating in air forms a substance Y. When hydrogen gas is passed over heated Y, it again changes back into X. Name the substances X and Y.
Balance the following chemical equations and state whether they are exothermic or endothermic.
A silver article generally turns back when kept in the open for a few days. The article when rubbed with toothpaste again starts shining.
(i) Why do they turn black? Name the phenomenon involved.
(ii) Name the black substance formed and write its formula.
Aluminium is a reactive metal but is still used for packing food articles. Why?
State one example each characterised by the following along with the chemical equation:
(i) Change in state (ii) Evolution of gas (iii) Change in temperature
Write balanced chemical equations for the following and identify the type of chemical reactions.
(i) Hydrogen iodine on reacting with chlorine gas gives iodine and hydrochloric acid.
(ii) Methane gas burns in oxygen of air to form carbon dioxide and water.
(iii) On passing electric current through molten aluminium oxide, it decomposes to form aluminium metal and oxygen gas.
Balance the following chemical equations. Write the symbols of physical states of all the reactants and the products.
Write chemical equations for the reactions taking place when
(i) Magnesium reacts with dilute HNO3
(ii) Sodium reacts with water.
(iii) Zinc reacts with dilute hydrochloric acid.
What happens when a piece of
(i) zinc metal is added to copper sulphate solution?
(ii) aluminium metal is added to dilute hydrochloric acid?
(iii) silver metal is added to copper sulphate solution? Also, write balanced chemical equation if the reactions occurs.
A housewife wanted her house to be white washed. She bought 10 kg of quicklime from the market and dissolved in 30 L of water. On adding lime to water, she noticed that the water started boiling even when it was not being heated. Give reason for her observation. Write the corresponding equation and name the product formed.
Write a balanced chemical equation for each of the following reactions and also classify them.
(i) Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
(ii) A piece of sodium metal is added to absolute ethanol to form sodium ethoxide and hydrogen gas.
(iii) Hydrogen sulphide gas reacts with oxygen gas to form solid sulphur and liquid water.
On adding a drop of barium chloride solution to an aqueous solution of sodium sulphite, white precipitate is obtained.
(i) Write a balanced chemical equation of the reaction involved.
(ii) What other name can be given to this precipitation reaction?
(iii) On adding dilute hydrochloric acid to the reaction mixture, white precipitate disappears. Why?
(i) Classify the following reactions into different types.
(a)
(b)
(c)
(ii) Which of the above reaction(s) is/are precipitation reaction(s)? Why is it so called?
State the type of chemical reactions with chemical equations that take place in the following.
(i) Magnesium wire is burnt in air.
(ii) Electric current is passed through water.
(iii) Ammonia and hydrogen chloride gases are mixed.
On heating blue coloured powder of copper (II) nitrate in a boiling tube, copper oxide (black), oxygen gas and a brown gas X is formed.
(i) Write a balanced chemical equation of the reaction.
(ii) Identify the brown gas X evolved.
(iii) Identify the type of reaction.
(iv) What could be the pH range of the aqueous solution of the gas X?
When solutions of silver nitrate and sodium chloride are mixed, a white precipitate forms. The ionic equation for the reaction is
(i) (a) What is the name of the white precipitate?
(b) Is it a soluble or insoluble compound?
(ii) Is the precipitation of silver chloride a redox reaction?
What is a reduction reaction? Identify the substances that are oxidised and the substances that are reduced in the following reactions:
State reason for the following
(i) Small amount of acid is added to water during electrolysis of water.
(ii) When ammonium chloride is dissolved in water in a test tube, the test tube becomes cold.
(iii) Paint is applied on iron articles.
(i) Why iron corrodes but aluminium does not?
(ii) Write the chemical name and the formula of the brown gas produced during thermal decomposition of lead nitrate.
(iii) What is the general name of the chemicals which are added to fat and oil containing foods to prevent the development of rancidity?
(i) Give an example for a combination reaction which is exothermic.
(ii) Identify the oxidising agent and reducing agent in the following reaction.
(iii) Name the phenomenon due to which the taste and smell of oily food changes when kept for a long time in open. Suggest one method
to prevent it.
There are different types of chemical reactions according to around us or being carried out for the benefit of mankind, e.g. combination reactions, decomposition reactions, displacement reactions, precipitation reaction, reduction-oxidation (redox) reactions, photochemical reactions etc.
Now, answer the following questions:
(i) Combustion of coke is a combination reaction. CO2 is not a pollutant. Then why is the combustion of coke harmful?
(ii) Which reaction followed by two combination reactions are involved in whitewash of walls?
(iii) Give one use of tin plating in daily life.
(iv) How photochemical reactions have played an important role in photography?
Identify the type of chemical reaction taking place in each of the following.
(i) Barium chloride solution is mixed with copper sulphate sodium and a white precipitate is observed.
(ii) On heating copper powder in air in a China dish, the surface of copper powder turns black.
(iii) On heating green coloured ferrous sulphate crystals, reddish brown solid is left and smell of a gas having odour of burning sulphur
is experienced.
(iv) Iron nails when left dipped in blue copper sulphate solution become brownish in colour and the blue colour of copper sulphate fades
away.
(v) Quicklime reacts vigorously with water releasing a large amount of heat.
You are provided with two containers made up of copper and aluminium. You are also provided with solutions of dil. HCl, dil. HNO3, ZnCl2 and H2O. In which of the above containers, these solutions can be kept?
(i) Identify the type of reactions taking place in each of the following cases and write the balanced chemical equations for the reactions.
(a) Barium chloride solution is mix, with copper sulphate solution and a white precipitate is obtained.
(b) On heating copper powder in air, the surface of the copper powder turns black.
(ii) What happens when hydrogen gas is passed over the heated copper oxide? Write the chemical equation involved in this reaction.
Define rancidity. What kind of substances are used to prevent rancidity? Explain any three methods to prevent rancidity.
What change in colour is observed when white silver chloride is left exposed in sunlight? State the type of chemical reaction in this change.
When the powder of a common metal is heated in an open China dish, its colour turns black. However, when hydrogen is passed over the hot black substance so formed, it regains its original colour. Based on the given information, answer the following questions.
(i) What type of chemical reaction takes place in each of the two given steps? Write balanced chemical equations for both reactions.
(ii) Name the metal initially taken in the powdered form.
Bhawna took a pale green substance A in a test tube and heated it over the flame of a burner. A brown coloured residue B was formed along with the evolution of two gases with burning smell of sulphur. Identify A and B. Write the chemical reaction involved.
In an experiment, solid calcium oxide was taken in a container and water was added slowly to it.
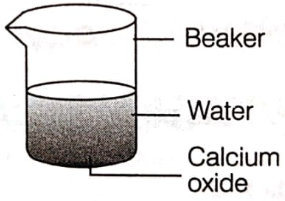
(i) State the two observations made in the experiment.
(ii) Write the name and chemical formula of the product formed.
In a school laboratory, a student wants to study the effect of heat on the ferrous sulphate crystals in a boiling tube. State the condition he is likely to draw on the basis of his observations.
The following given statements have been written to study the type of reaction, but they are not correct, you have to rewrite them making necessary corrections.
(i) To study the displacement reaction, copper turnings were added in the zinc sulphate solution.
(ii) To study the double displacement reaction, solid sodium sulphate was mixed with solid barium chloride and a yellow colour precipitate
was obtained.
We have often seen that oily food if not used within a limited time gives bad taste, smell and becomes unfit for consumption. This is due to oxidation of oils and fats present in the food. Now, answer the following questions:
(i) On marriages or other celebrations, a lot of food goes waste. What methods do you suggest to prevent wastage?
(ii) Often preservatives are added to certain foodstuff, so that they can stay consumable for a long time but these preservatives are
chemicals which may be harmful. What alternative do you suggest?
(iii) What method of preservation of food items should be followed at home?
Seema bought a chips packet and opened it. Suddenly her friend Shanu came. She started playing and forget to eat the chips. On the next day, when she eats the chips, she felt the taste was not good and her health fell down. She told her mother to take her to a doctor. The doctor told them this is because of eating rancid chips. He gave medicines, by which Seema became well within a few days.
Read the above passage and answer the following questions.
(i) Why do chips remain fresh for a longer time in a sealed packet?
(ii) Why did chips of an open packet become rancid?
(iii) What value of you infer from this passage?