At the end of spermatogenesis, sperms heads become embedded in the sertoli and finally released from the seminiferous tubules by the process called
1. Spermiogenesis
2. Spermateliosis
3. Spermiation
4. Androgenesis
If the embryoblast is removed from the developing blastocyst, which of the following part will develop as usual ?
1. Head part of embryo
2. Embryonic gut
3. Placenta
4. Caudal part of embryo
Mark the type of hepatits which can be spread through fecal oral route
1. Hepatitis - A
2. Hepatitis - B
3. Hepatitis - C
4. All of these
Yolk sac is formed by
1. Somatopleuric extraembryonic mesoderm and ectoderm
2. Somatopleuric extraembryonic mesoderm and trophoectoderm
3. Splachnopleuric extraembryonic mesoderm and endoderm
4. Splachnopleuric extraembryonic mesoderm and ectoderm
First polar body has
1. Large amount of cytoplasm and haploid set of chromosome
2. Small amount of cytoplasm and haploid set of chromosome
3. Large amount of cytoplasm and diploid set of chromosome
4. Small amount of cytoplasm and diploid set of chromosome
Which of the following is removed during maturation of insulin?
1. A chain
2. B chain
3. C chain
4. Disulphide bond
Basmati rice is distinct for its unique aroma and flavour. How many documented varieties of Basmati are grown in India ?
1. 27
2. 270
3. 13
4. 230
The separated DNA fragments can be visualised
1. Only after staining with a compound ethidium bromide
2. Under visible light
3. Under UV light
4. Both (1) & (3)
Cocaine, commonly called as coke or crack is obtained from
1. Papaver somniferum
2. Erythroxylon
3. Cannabis sativa
4. Claviceps purpura
Cancer cells differ from normal cells by the
1. Breakdown of regulatory mechanism
2. Loss of the property of contact inhibition
3. Attaining the property of contact inhibition
4. Both 1 & 2
MALT (Mucosa-associated lymphoid tissue) constitutes:
1. 25 percent of the lymphoid tissue in the human body
2. 50 percent of the lymphoid tissue in the human body
3. 75 percent of the lymphoid tissue in the human body
4. 90 percent of the lymphoid tissue in the human body
Mark the incorrect statement with respect to AIDS:
| 1. | AIDS is not spread by mere touch. |
| 2. | Time lag between infection and the appearance of AIDS symptoms is just 5-6 months only. |
| 3. | It is caused by a retrovirus. |
| 4. | HIV enters body cells by receptor-mediated endocytosis. |
Infertility due to very low sperm count in the ejaculates should opt for which of the following assisted reproductive technologies ?
1. GIFT
2. IUI
3. Testicular sperm extraction
4. Microsurgical epidymal sperm aspiration
Which one is not applicable with respect to control experiment of Miller ?
1. Ratio of is 1:1:2
2. Presence of water vapour
3. Created electric discharge in closed flask 800C
4. Both 1 and 3
Which of the following is not an example of analogous structure ?
1. Eye of Octopus and mammal
2. Flippers of penguins and dolphin
3. Sweet potato and potato
4. Mouth parts of cockroach and mosquito
Mark the correct statement with respect to industrial melanism:
| 1. | Lichens can be used as an industrial pollution indicator. |
| 2. | Low count of melanic moths are found in rural areas where industrialisation did not occur. |
| 3. | Neither grey nor the dark variety of moths has been completely wiped out. |
| 4. | All of these |
Agriculture and human settlement started about
| 1. | 75000 years ago | 2. | 18000 years ago |
| 3. | 10000 years ago | 4. | 1.5 mya |
Mark the immunoglobulin which gives passive immunity to newly born baby via material milk
1. IgG
2. IgA
3. IgE
4. IgM
Ringworms which is one of the most common infectious disease in man is caused by
1. Epidermophyton
2. Trichophyton
3. Microsporum
4. All of these
Slightly prognathous face, walked upright, low brow bridge receding jaws, high domed heads adapted for cold environment, cannibals had a religion buried dead ones with flower and tools are the characteristics of
1. Cromagnon
2. Neanderthal man
3. Java man
4. Heidelberg man
Most serios or even fatal type of malaria is caused by
1. P vivax
2. P. falciparum
3. P ovale
4. P malanae
Which of the following cross often helps to overcome inbreeding depression ?
1. Inbreeding
2. Outcrossing
3. Cross breeding
4. Interspecific hybridisation
Which of the following virus mainly associated with warts over the skin and mucosal surface of external genitalia and perianal area ?
1. Human papiloma virus
2. Hepatitis B virus
3. Human immuno deficiency virus
4. Epstein Barr virus
Major histocompatibility complex class-II is present with
1. Almost all the cells without exception
2. All the viruses
3. Macrophage B cells and dendritic cells
4. All enucleated cells
Secondary immune response is more rapid, and lasts much longer than primary response is due to
| 1. | Antigen presenting cells |
| 2. | Memory cells |
| 3. | T cyclotoxic cells |
| 4. | T helper cells |
Which of the following can be termed as age of jawless vertebrates?
1. Ordovician
2. Silurian
3. Cambrian
4. Devonian
Number of paratope/s present in IgM is/are
1. Two
2. One
3. Four
4. Ten
Which of the following restriction enzyme produces blunt or flush end ?
1. Sma - I
2. Hae - III
3. Bam - HI
4. Both 1 and 2
Mark the hormone-releasing IUDs which suppress endometrial changes in cervical mucus, cause anovulation and insufficient luteal activity.
1. Lippes Loop
2. Multiload 375
3. LNG - 20
4. Vaults Cap
Which of the following about AIDS virus is incorrect ?
| 1. | AIDS virus is RNA virus |
| 2. | The major cell infected by HIV is Helper T lymphocyte that bears receptor sites |
| 3. | The attachment of virus of receptor site is by the help of GP-4 T on the protein coat of virus |
| 4. | The genome of HIV consists of two identical molecules of single stranded RNA |
Transverse binary fission is exemplified by
1. Euglena
2. Ceratium
3. Paramecium
4. Plasmodium
In case of natural method of contraception, like Lactational Amenorrhoea, it serves for a maximum period of
| 1. | One week |
| 2. | One month |
| 3. | Up to the initiation of menstrual flow |
| 4. | 6 months |
The reason to legalise conditional MTPs by the government of India is
| 1. | To decrease the population growth rate |
| 2. | To check indiscriminate and illegal male foeticide |
| 3. | To check illegal female foeticides which are reported too high in India |
| 4. | To check pregnancies |
Which of the following is not a natural method for birth control ?
| 1. | Periodic abstinence |
| 2. | Coitus interruptus |
| 3. | Lactational amenorrhoea |
| 4. | Vaults |
Hugo de Vries gave the theory of mutation. He conducted his experimental on
1. Oenothera lamarkiana
2. Pisum sativum
3. Evening primrose
4. Both 1 and 3
During course of evolution, the land reptiles which went back into water to evolve into fish-like reptiles, about 200 mya would refer to
1. Icthyosaurs
2. Tyrannosaurus
3. Pelycosaur
4. Therapsid
Antitoxin consists of
1. Antibodies
2. Toxoid
3. Antibiotics
4. Live attenuated pathogen
Which kind of disadvantage would be present with Bovine insulin (derived from cattle) as compared to human insulin ?
1. Absorption from intestine is slow
2. Possible immune response from the receiver
3. Poisonous
4. All the above are disadvantages
Which organisation in India deals with the safety of introducing GM- organisms for public services?
1. GEAC
2. CFTRF
3. NII
4. CDRI
Find the wrong match.
1. Wheat → Pusa Shubra
2. Cauliflower → Pusa Snowball K-1
3. Chilli → Pusa Sadabahar
4. Brassica → Pusa Swarnim
The most practical means to improve public health and enriching foods with vitamins and minerals would include.
1. Micropropagation.
2. Use of single cell proteins.
3. Biofortification.
4. Developing pest resistant crops
In high yielding hybrid crop varieties,to exploit hybrid vigour, the farmers need to purchase fresh hybrid seed every year because :-
1. They are not allowed to grow their own seed
2. Hybrid vigour is lost due to inbreeding depression
3. It is cheaper to purchase fresh seed
4. None
Biological Pest Control methods are more preferably used because-
1. Chemical pesticides are discriminate
2. Predators work through check and balance
3. Predators are indiscriminate
4. All of above
Match the following list of bioactive substances and their roles :
Bioactive Substance-
(i) Statins (ii) Cyclosporin A (iii) Streptokinase (iv) Lipase
Role-
(A) Removal of oil stains
(B) Removal of clots from blood vessels
(C) Lowering of blood cholesterol
(D) Immuno-suppressive agent
Choose the correct match:
1. i B, ii C, iii A, iv D
2. i D, ii B, iii A, iv C
3. i B, ii A, iii D, iv C
4. i C, ii D, iii B, iv A
In hydrarch succession pioneer is phytoplankton and climax is forest (mesic). Given below is name of seral stages, arrange them in accordance with their appearance.
A. Submerged free floating plants
B. Reed – swamp stage
C. Marsh meadow stage
D. Submerged plant stage
E. Scrub stage
Option
1. A, B, C, D & E
2. B, A, C, D & E
3. D, A, C, B & E
4. D, A, B, C & E
Ecosystem requires constant supply of energy to synthesise the molecules they require for;
1. Storage, as when needed can be utilized
2. To counteract the universal tendency towards increasing disorderliness
3. As per first law of thermodynamics
4. None is correct
Further development of zygote depends upon?
1. Type of life cycle followed by organism.
2. Environment in which that zygote develops.
3. Behaviour of that organism.
4. Both (1) and (2)
"All organisms have to reach a certain stage of maturity and growth, before they can reproduce sexually”. How many of the following is true for above statement?
(I) It is known as juvenile phase.
(II) It is known as vegetative phase in plants.
(III) It is of different duration in different organisms.
(IV) It is of same duration in different organisms.
Options :
1. 4
2. 3
3. 2
4. 1
The above given diagram is an enlarged view of one microsporangium of a matured anther. Identify A, B and C
1. A - Middle layer, B - Endothecium, C – Tapetum
2. A - Endothecium, B - Tapetum, C - Middle layer
3. A - Endothecium, B - Middle layer, C – Tapetum
4. A- Tapetum, B - Middle layer, C – Endothecium
The inner wall of pollen grain:
| 1. | Is thin, continuous and pecto-cellulosic and is called intine |
| 2. | Comes out in the form of pollen tube through germpore |
| 3. | Is thick and consists of sporopollenin |
| 4. | (1) and (2) |
During the formation of embryo sac from megaspore mitotic divisions occurs. These mitotic divisions are:
1. Strictly free nuclear
2. Strictly cellular
3. Strictly reduction
4. Strictly cytoplasmic
Which of the following statements is false about filiform apparatus?
| 1. | The synergids have special cellular thickenings at the micropylar tip called filiform apparatus |
| 2. | It plays an important role in guiding the pollen tubes into the synergid |
| 3. | Both (1) and (2) |
| 4. | Pollen tube stimulates the formation of filiform apparatus |
Which of the following plants produce(s) chasmogamous and cleistogamous flowers?
1. Viola (Commonpansy)
2. Oxalis
3. Commelina
4. All of the above
Find out the correct option:
1. Among animals, insects, particularly bees are the dominant biotic pollinating agents
2. Often flowers of animal- pollinated plants are specially adapted for particular species of animals
3. In some species floral rewards like edible nectar, pollen grains, shelter for egg laying are given to pollinating animals
4. All the above are correct
Which of the following is not correct?
1. As the seed matures, its water content is reduced and seeds become relatively dry(10-15% moisture by mass)
2. The seed dormancy is the internal or innate inhibition of generation of normal or viable seeds
3. Embryo in dormant seed shows higher rate of general metabolic rate
4. Because of dormancy seeds remain viable for longer period and can be stored
Which of the following statements is false?
1. Some 2000 years old viable seeds of Pheonix dactylifera were discovered during archaeological excavation of King Herod's palace near Dead Sea
2. Record of 10,000 years of dormancy of seeds has been estimated in Lupinus arcticus
3. The number of seeds in each fruit in case of orchid and some parasitic forms like Orobanche and Striga is one
4.Many fruits have evolved mechanisms for dispersal of seeds
Match the columns with respect to the process of translation:
| Column I | Column II | ||
| (a) | UTR | (i) | Catalyst |
| (b) | rRNA | (ii) | Template |
| (c) | mRNA | (iii) | Reads the genetic code |
| (d) | tRNA | (iv) | For efficiency |
| (a) | (b) | (c) | (d) | |
| 1. | (i) | (ii) | (iii) | (iv) |
| 2. | (iv) | (i) | (ii) | (iii) |
| 3. | (iv) | (iii) | (ii) | (i) |
| 4. | (ii) | (iii) | (iv) | (i) |
Match items in Column-I with Column-II:
Column-I Column-II
a. Bioinformatics (i) Non-coding DNA
b. Satellite-DNA (ii) Genes which expressed as RNA
c. ETS (iii) Coding and non-coding DNA sequencing
d. Sequence annotation (iv) Computational techniques for genome sequencing
1. a(iv), b(i), c(iii), d(ii)
2. a(iv), b(iii), c(i), d(ii)
3. a(iii), b(iv), c(ii), d(i)
4. a(iv), b(i), c(ii), d(iii)
Consider the following statements regarding DNA fingerprinting:
i. The technique was initially developed by Alec Jeffreys.
ii. Hybridisation using labeled VNTR probe.
iii. Sensitivity of the technique has been increased by the use of PCR.
iv. Sequences used for DNA fingerprinting generally code for many proteins.
v. Monozygotic twins have identical DNA fingerprints.
1. All statements are correct
2. Only '4' is incorrect
3. 4 and 5 are incorrect
4. 1, 3, 4, and 5 are correct
Gametes are always haploid. What percent of sperms and ova of grasshopper lack 'x' body respectively?
1. 50% and 50%
2. 50% and 0%
3. 0% and 0%
4. 0% and 50%
Test cross is:
1. between two homozygous genotype for different traits of same character
2. Cross between two heterozygous genotype of different character
3. Cross between one homozygous and one heterozygous genotype for different traits of same character
4. Cross between one heterozygous and homozygous of similar traits of same character
Which of the following scientist is responsible for the synthesis of protein in a cell-free system?
1. Har Gobind Khorana
2. Marshall Nirenberg
3. Severo Ochoa
4. Frederic Sanger
Read the following four statements (A-D)
A. The characters blend in heterozygous condition.
B. Change in a single base pair of DNA does not cause mutation.
C. Cancer cells commonly show chromosomal aberrations.
D. In insect, sex chromosomes in male are ZZ and in females are ZW.
How many of the above statement is/are right?
1. Two
2. Three
3. Four
4. One
How many of the following statements is/are correct for a polygenic inheritance?
| 1 | They show uniformity. |
| 2 | Controlled by three or more genes |
| 3 | It is not influenced by the environment. |
| 4 | In polygenic inheritance phenotype reflects the contribution of dominant allele only. |
Options :
1. 1 and 2
2. 2, 3 and 4
3. 1, 3 and 4
4. only 2
Which is not true for haplodiploid sex determination?
1. It is reported in the honeybee.
2. In this male produces sperms by meiosis.
3. They do not have fathers and thus cannot have sons.
4. In this unfertilized egg develops as a male by means of parthenogenesis.
The deletion in chromosome number 16 results in which of the following genetic disorder?
1. Pleiotropy
2. Quantitative problem of RBC’s
3. Qualitative problem of RBC’s
4. Hypertrichosis
Conditions of a karyotype 2n ± 1 are observed in all except
1. Down syndrome
2. Turner’s syndrome
3. Phenyl ketonuria
4. Klinefelter’s syndrome
Choose the correct statement about recombination and linkage
1. Complete linkage results in higher non-parental gene combinations than parental type
2. Recombination results in the generation of parental gene combinations
3. Genetic maps are constructed by using the frequency of recombination between gene pairs
4. T.H. Morgan constructed the first chromosome map
Which of the following is not the property of a molecule that can act as genetic material?
1. Able to replicate
2. Able to mutate
3. Chemically and structurally stable
4. Should express as dominant characters.
Distribution of newly synthesized DNA in the chromosomes is by semi-conservative means is experimentally shown by Taylor et al by using
1. Radioactive adenosine
2. Radioactive thymidine
3. Radioactive guanosine
4. Radioactive cytidine
During DNA replication, two strands of DNA cannot be separated in their entire length in one step because?
1. Due to high energy requirement
2. Due to complementary base pairing
3. Due to antiparallel nature of DNA strand
4. Due to absence of enzyme DNA polymerase
If due to mutation, both the strands of DNA start transcribing, which of the following will not be the consequence of this?
1. Complicate the genetic information transfer machinery
2. One segment of DNA would code for two different protein
3. Will prevent translation
4. Will result in polyploidy
In nature, a given habitat has enough resources to support a maximum possible number, beyond which no further growth is possible. The limit is known as
1. Environmental resistance
2. Intrinsic rate of natural increase
3. Carrying capacity
4. Exponentially growth
Fitness of one species (measured in terms of intrinsic rate of increase) is significantly lower in the presence of another species during the interaction between
1. Barnacles and whale
2. Abingdon tortoise and goats
3. Ophrys and bee
4. Sea anemone and clown fish
Which the following pairs is wrongly matched while remaining three are correct
1. Allen’s rule – Mammals with shorter ears and limbs in colder area
2. Expanding population – High number of individual at pre-reproductive stage
3. Population density increasing – (B + I) < (D + E)
4. Carrying capacity – Logistic growth.
Consider the following four statements (A–D), select the correct option stating which ones are true (T) and which ones are false (F)
| (A) | Organism at each trophic level depends on those at higher trophic levels for their energy demands. |
| (B) | Each trophic level has a certain mass of living material at a particular time called standing crop. |
| (C) | The amount of inorganic matter present in an ecosystem at a given time called standing state. |
| (D) | The number of trophic levels in a grazing food chain is not restricted |
| A | B | C | D | |
| 1. | T | T | F | F |
| 2. | F | T | F | T |
| 3. | T | F | T | F |
| 4. | F | T | T | F |
In any ecological pyramid, an organism can occupy how many trophic levels?
1. Only one trophic level
2. More than one trophic level simultaneously
3. Can occupy more than one trophic level but not simultaneously
4. Very difficult to say in a precise manner
Climax community is in a state of:
1. non-equilibrium
2. equilibrium
3. disorder
4. constant change.
Which of the following is an ecosystem service provided by a natural ecosystem?
1. Cycling of nutrients
2. Prevention of soil erosion
3. Pollutant absorption and reduction of the threat of global warming
4. All of the above
Which of the following is not correct with respect of control of vehicular air pollution in India?
1. Use of CNG as fuel
2. Not phasing out of old vehicles as they have better technology
3. Use of unleaded petrol, low-sulphur petrol and diesel
4. Use of catalytic converter
Which one of the following pairs is wrongly matched remaining three are correct?
|
|
Legislation |
Year |
|
1. |
Environment (Protection) Act |
1986 |
|
2. |
Air (Prevention and control of pollution)Act |
1981 |
|
3. |
Noise as on air pollutant |
1987 |
|
4. |
Water (Prevention and control of pollution) Act |
1984 |
DDT which is widely used, is responsible for which of the following effect
(A) Accumulates in human being as a result of biomagnifications
(B) Disturbs calcium metabolism in birds
(C) Responsible for decline in population of certain birds
(D) Acts as a signalling molecule in biochemical pathway
Select the correct option
1. I, II, IV is correct
2. I, II, III is correct
3. I, III, IV is correct
4. I, II, III, IV is correct
Consider the following four statements (A-D) related to Green house effect and Global warming and select the correct option stating which one are true (T) and which ones are false (F).
Statements
(A) It is responsible for maintaining average temperature at 18ºC
(B) CO2, CH4, CFC, N2O are green house gases
(C) Responsible for El Nino Effect
(D) Montreal protocol is for this
Options
A B C D
1. T T F F
2. F F T T
3. F T T F
4. T F F T
Which one of the following statement is totally wrong about greater biological diversity in tropics
1. Tropical latitude have remained relatively undisturbed for millions of years
2. Tropical environment is more less constant and predictable
3. Soil here is very fertile
4. More solar energy is available.
Which one of the following is not a major characteristic feature of biodiversity hot spots?
1. Large number of species
2. Abundance of endemic species
3. Large number of exotic species
4. Destruction of habitat
World Summit on sustainable development held in 2002 in
1. Rio de Janeiro
2. Japan
3. Johannesburg
4. London
The theory of spontaneous generation says that
1. Life originated from the decaying and rotting matter like straw , mud etc
2. Life came on the earth from outerspace
3. Life comes from pre-existing life only
4. Life started with the replication of self replicating metabolic capsules
MOET has not been practiced in
a. Cattle b. Sheep
c. Rabbits d. Poultry
1. b,c & d
2. b & d
3. d only
4. c only
Which of the following is correct to check the inbreeding depression ?
1. Artificial hybridisation
2. Cross breeding
3. Selected animal should be mated with unrelated superior animals of the same breed
4. Selected animal should be mated with unrelated superior animals of the different breed
Which of the following are edible marine fishes ?
1. Hilas, Catla, Sardines
2. Sardines, Mackerel, Rohu
3. Hilsa, Sardines, Mackerel
4. Mackerel, Pomfrets, common carp
The activation energies of two reactions are and with > .
f the temperature of the reacting system is increased from T1 to T2 .
The correct relation is:
1.
2.
3.
4.
In the following first order competing reaction –
The ratio of k1/k2 if only 50% of B will
have been reacted when 94% of A has been reacted is –
1. 4.06
2. 3.06
3. 2.06
4. 0.06
According to effective atomic number rule the central metal acquires :
1. Inert gas configuration
2. Duplet
3. Octet
4. Quartet
The correct name of 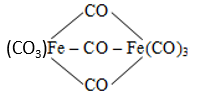 is
is
1. Tri--carbonyl bis (tricarbonyl iron (0)
2. Hexacarbonyl iron (III) -tricarbonyl ferrate (0)
3. Tricarbonyl iron (0) -tricarbonyl iron (0) tricarbonyl
4. Nonacarbonyl iron
Increasing order of 0 of the following complex is –
1. Cr(NH3)63+ < Cr(H2O)63+ < Cr(NO2)63–
2. Cr(H2O)63+ > Cr(NH3)63+ > Cr(NO2)63–
3. Cr(H2O)63+ < Cr(NH3)63+ < Cr(NO2)63–
4. Cr(NH3)63+ < Cr(NO2)63– < Cr(H2O)63+
The number of geometrical isomers of [Co(NH3)2(NO3)2] are :
1. 0 2. 2
3. 3 4. 4
Calculate the molal depression constant of a solvent which has freezing point 16.60 and latent heat of fusion 43 calories.
1. 3.3
2. 3.86
3. 2.9
4. 38.6
The van’t Hoff factor for a very dilute
solution of is
1. 9
2. 5
3. 2
4. 6
CsBr crystallizes in a body-centered cubic lattice. The unit cell length is 436.6pm. Given that the atomic mass of Cs u =133 and that of Br = 80 u and Avogadro number is , the density of CsBr is
1. 42.5 g/
2. 4.25 g/
3. 8.25 g/
4. 82.5 g/
According to Freundlich adsorption isotherm, at high pressure, the value of is -
1. Directly proportional to the pressure.
2. Inversely proportional to the pressure.
3. Directly proportional to the square of the pressure.
4. Independent of the pressure.
Activators are generally metal ions, the
catalytic activity of amylase increases in
presence of
1. Na+
2. Co2+
3. Cu2+
4. Mn2+
The correct statement among the following is:
| 1. | The rate of reaction cannot be understood from the Ellingham diagram. |
| 2. | During the formation of metal oxide \(\Delta S\) becomes negative and \(\Delta G\) becomes positive resulting in a positive slope. |
| 3. | There is an abrupt change in the slope of the Ellingham line when a change in phase (s→l) or (l→g) takes place. |
| 4. | All of the above. |
Sodium dissolved in Ammonia has Blue
colour due to
1. Solvated Sodium
2. Amide Ion
3. Solvated electron
4. Lone pair of electrons on Nitrogen in NH3
molecule
Ammonia is not a product in the
1. Hydrolysis of nitrolim
2. Hydrolysis of Aluminium nitride
3. Decomposition of Ammonium nitrite
4. Hydrolysis of urea
Phosphine can not be produced by the following reaction:
1. White P is heated with NaOH.
2. Red P is heated with NaOH.
3. Ca3P2 is heated with water.
4. Phosphorus trioxide is boiled with water.
Which of the following alkyl halide is used as an ethylating agent?
1. CH3I
2. C2H5Cl
3. C2H4Br2
4. C2H5OH
A gas that comes out when ethyl alcohol is heated with methyl magnesium iodide, is -
| 1. | Methane | 2. | Ethane |
| 3. | Carbon dioxide | 4. | Propane |
Identify A and B in the following reaction
1. A = aqueous KOH; B = moist Ag2O
2. A = alcoholic KOH ; B = aqueous NaOH
3. A = aqueous NaOH ; B = AgNO2
4. A = AgNO2 ; B = KNO2
The alcohol expected to have the lowest pKa value among the following is -
1. Ethanol
2. 2-Fluoro ethanol
3. 2,2,2-Trifluoroethanol
4. 2-Chloroethanol
 \(\xrightarrow[(ii). \ H_{3}O^{+}]{(i). \ OH^{-}}\)
\(\xrightarrow[(ii). \ H_{3}O^{+}]{(i). \ OH^{-}}\)
| 1. |  |
2. |  |
| 3. |  |
4. |  |
If NaCl is doped with mol% of , the number of cation vacancies will be -
1.
2.
3.
4. 2
A compound that shows Schottky as well as Frenkel defect among the following is -
1. ZnS
2. AgBr
3. NaCl
4. AgCl
Which of the following is correct for both fluorite and anti-fluorite structure?
(1) Cations are present in alternate tetrahedral void
(2) Cations constitute the lattice
(3) No. of formula units in 1 unit cell is 4
(4) Oxides of alkali metals have fluorite structure whereas their fluorides have anti-fluorite structure
0.01 m aqueous solution of freezes at for water is 1.86 K kg . The apparent percentage of dissociation will be
(1) 77.7 (2) 59.8 (3) 47.7 (4) 22.3
What will happen to the elevation in boiling point of a solution, if the weight of the solute dissolved is doubled but the weight of the solvent taken is halved?
1. Elevation in boiling point will become four times
2. Elevation in boiling point will become double
3. No change in elevation in boiling point
4. Elevation in boiling point will become half.
The free energy change for the decomposition reaction
The minimum potential difference needed to reduce will be
1. 1.874 V
2. -2.487
3. 960 V
4. 2.487 V
If hydrogen electrodes dipped in two solutions of pH = 4 and pH = 6 are connected by a salt bridge, the emf of the resulting cell is -
1. 0.177 V
2. 0.3 V
3. 0.118 V
4. 0.104 V
If equivalent conductance of 1 M is 12.8 and if the conductance of acetate ion and ion at infinite dilution are 42 and 288.42 respectively, its degree of dissociation is
1. 39%
2. 3.9%
3. 0.35%
4. 0.039%
If electrode is diluted 100 times, then the change in emf is
1. Increase of 59 mV
2. Decrease of 59 mV
3. Increase of 29.5 mV
4. Decrease of 29.5 mV
75% of the original amount of a reactant was added to the reaction mixture after 40 minutes. What percentage of the total amount will be present after 60 minutes, given that half-life period of the reaction is 20 minutes?
(1) 25% (2) 12.5% (3) 28.5% (4) 100%
Among the following options, the gas that will be more readily adsorbed on the surface of charcoal is:
1.
2.
3.
4.
A current of 9.65 ampere flowing for 10 minutes deposits 3.0 g of the metal which is trivalent. The atomic mass of the metal is
1. 30
2. 150
3. 90
4. 289.5
The incorrect statement among the following is -
1. is more stable than
2. is less stable than but is more stable than
3. is less stable than
4. Pb(II) is a good reducing agent while Sn(II) is not
Which of the following statements is incorrect?
1. Nitric oxide becomes brown when released in air
2. solid phosphorous pentachloride exhibits some ionic character
3. Ammonia is a good complexing agent
4. gets hydrolysed readily
which of the following halides is most acidic?
1.
2. Sb
3. Bi
4.
Mohr salt,
(1) Mohr salt is a double salt while ferrous sulphate is a single salt
(2) Mohr salt is not hygroscopic but is hygroscopic
(3) Mohr salt contains only ferrous ions whereas ferrous sulphate contains some ferric ions
(4) Mohr salt solution can be titrated even in the absence of
\((i) Zr + 2I_2 \xrightarrow{870K} ZrI_4 \xrightarrow{2075K} Zr + 2I_2 \)
\((ii) Ni + 4CO \xrightarrow{330K} Ni(CO)_4 \xrightarrow{450K} \underset{(pure)}{Ni} + 4CO \)
The processes (ii) and (i) above, respectively, are :
1. Mond's process; Van Arkel process
2. Van Arkel process; Mond's process
3. Goldschmidt process; Mond's process
4. Mond's process; Goldschmidt process
The correct statement among the following is:
| 1. | If ∆0 < P, low spin state is more stable. |
| 2. | CO is a very weak ligand. |
| 3. | The colour of a complex depends only on the nature of metal ion. |
| 4. | CO is a weak base but strong ligand. |
The complex that does not have a tetrahedral shape is:
1.
2.
3.
4.
If Compound A (C₄H₈) is treated with H₂O/H₂SO₄ and forms an optically inactive C₄H₁₀O, what is the structure of A?
| 1. | CH3CH2CH=CH2 | 2. | CH3CH=CHCH3 |
| 3. | (CH3)2C=CH2 | 4. |
The most reactive compound towards the nucleophilic addition reaction is:
| 1. | Benzaldehyde | 2. | p-Tolualdehyde |
| 3. | p-Nitrobenzaldehyde | 4. | Acetophenone |
The product formed, when ethyl formate is treated with excess of n-propylmagnesium bromide and the product is acidified is
(1) Butanal (2) Heptan-4-ol
(3) Butanol (4) Heptanal
\(A \left(\right. C_{3} H_{9} N \left.\right) \) reacts with benzenesulfonyl chloride to give an insoluble salt in alkali.
The structure of compound (A) will be:
| 1. | \(\mathrm{CH_3CH_2CH_2NH_2} \) | 2. | \(\mathrm{CH_3NHCH_2CH_3}\) |
| 3. |  |
4. | None of the above |
Cmpound 'A' reacts with limited supply of oxygen gives compound B. B on reaction with hot water gives C and D. D is also formed by reaction of 'A' with aqueous NaOH while C is tribasic acid. D is also called phosphorated hydrogen. compound 'A' is
(1) (2) (3) (4)
The number of geometrical isomers are possible for the complex
(1) zero (2) 2 (3) 4 (4) 3
A particle having charge when placed at a point in an electric field experiences a force . The electric field at that point-
1.
2. >
3. <
4. May be any of the above depending on the source of the field.
A proton and an particle after being accelerated by same potential difference, are subjected to a uniform transverse electric field. Then one can conclude that
(1) Proton will deflect more
(2) particle will deflect more
(3) Both will be deflected equally but in opposite directions.
(4) Both will be deflected equally but in the same direction
When a metallic sphere is placed between the plates of a charged capacitor. Which of the following figures best represents the electric field lines of the system?
(1)
(2)
(3)
(4)
In the situation shown in figure, S is an imaginary closed surface. At point P (on the surface) electric field is produced by
(1) only
(2) only
(3) only
(4)
Two metallic shells with charges q and -q are kept in air with their centre distance r. The magnitude of electrostatic force between them is
(1) Equal to
(2) Less than
(3) More than
(4) Any of the above depending on the ratio of the radii of the shells
The electric flux through a hemispherical curved surface of radius R, placed in a uniform electric field of intensity E perpendicular to its circular plane is
(1) 2RE
(2) 2E
(3) E
(4) Zero
Electric potential (in volts) in a region is given by . The magnitude of electric field in the region is (where x, y and z are in metres)
1. 6 V 2. 7 V
3. 11 V 4. 5 V
In the circuit shown in figure, the potential difference is
(1) -12 V
(2) -6 V
(3) 12 V
(4) 6 V
The charge on the plates of the capacitor in a steady-state will be:
1. \(3~\mu\text{C}\)
2. \(9~\mu\text{C}\)
3. \(27~\mu\text{C}\)
4. \(36~\mu\text{C}\)
Two equal and opposite charges are placed are placed at a certain distance apart. Force between them has a magnitude F. If 8% of one charge is transferred to other, then new force between them will be nearly.
(1) (2)
(3) (4)
In the circuit shown in figure, the effective resistance of the system between the points A and B is 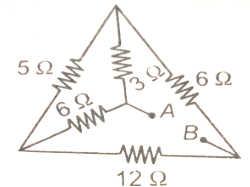
| 1. | 6 | 2. | 9 |
| 3. | 11 | 4. |
In the network shown in figure the current in in
(1) (2) (3) (4) zero
In the circuit shown in figure, the current through the 2 resistor will be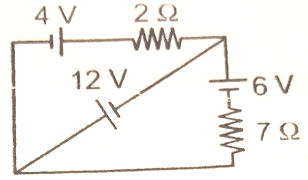
1. 8 A
2. 6 A
3. 11 A
4. 5 A
When a bulb of resistance 96.8 and a heater of resistance 387.2 are connected in series across a source of 220 V, the total power consumed in the circuit will be
(1) 80 W (2) 20 W (3) 100 W (4) 625 W
Electrostatic potential in a region is given by V = (3x - 2y + 4z) volt. The electric field in this region is
(1) Uniform
(2) Non-uniform varying with x
(3) Non-uniform varying with x and y both
(4) Non-uniform varying with x, y and z
In the circuit shown, the electric current l is
(1) (2) (3) (4)
A resistor of resistance \(R\) is connected to an ideal battery. If the value of \(R\) is decreased, then the power dissipated in the resistor will:
1. increase
2. decrease
3. remain unchanged
4. first increase and then decrease
A particle having charge q moves in a uniform magnetic field of magnitude B in a circular path such that its time period is T. If its charge is doubled without changing its mass, then its new time period will be
(1) (2) 2T (3) (4) T
| 1. | \(N\) is small | 2. | \(B\) is small |
| 3. | \(A\) is small | 4. | \(C\) is small |
In a potentiometer arrangement, a cell of emf 5 V gives a null point at 35 cm length of wire. If the cell is replaced by another cell, null point shifts to 56 cm. The emf of the new cell will be
(1) 6 V (2) 8 V (3) 6.5 V (4) 7.5 V
A bar magnet when allowed to oscillate in horizontal plane at a place, oscillated with a time period T. If the same magnet is allowed to oscillate in magnetic meridian, it oscillates with a time period . The dip angle at that place must be
(1) 30 (2) 60 (3) 45 (4) 90
A steel wire has magnetic moment M. If it is bent from middle at angle of 90, the new magnetic moment will be
(1) (2) (3) (4)
In the situation shown in figure, the current is decreasing at a constant rate of 2 A. The potential difference between the points A and B, when current in the circuit i = 5 A, will be -
(1) 27 V (2) 47 V (3) 3 V (4) 2 V
A solenoid has self inductance L. If number of turns in it are doubled without changing the number of turns per unit length, then its new self inductance will be
(1) 3L (2) 2L (3) 4L (4)
If a magnet is allowed to fall through a long cylindrical conductor of very large length, then its acceleration
(1) First increases, then becomes constant
(2) First decreases, then becomes constant
(3) Reduces to zero gradually
(4) Increases continuously
The magnetic flux through the primary of a transformer varies according to the relation . If number of turns in primary and secondary of the transformer are 10 and 1000, then secondary emf at t = 1 s will be
(1) 10 V (2) 100 V (3) 1000 V (4) 10000 V
In a circuit the current is given by i = (6A) sin 2t where t is in seconds. The mean value of current from t = 0 to t = 2 s will be
(1) (2) (3) (4) zero
In the circuit the rms current supplied by the source is
(1) 50 A (2) 100 A (3) 141.4 A (4) 200 A
A bulb of 220 volt and 200 Watt is connected across 110 volt circuit. The percentage reduction in power is
(1) 25% (2) 50% (3) 75% (4) 100%
| 1. | \(\oint_S \vec{E} \cdot \overrightarrow{d S}=\frac{1}{\varepsilon_0} \int_V \rho d V\) |
| 2. | \(\oint_S \vec{B} \cdot \overrightarrow{d S}=\frac{m}{\mu_0}\) |
| 3. | \(\oint_S \vec{E} \cdot \overrightarrow{d l}=-\frac{d}{d t} \int_S \vec{B} \cdot \overrightarrow{d S}\) |
| 4. | \(\oint_S \vec{H} \cdot \overrightarrow{d S}=\int_C\left(\vec{J}+\frac{d}{d t}\left(\varepsilon_0 \vec{E}\right)\right) \cdot \overrightarrow{d S}\) |
In an electromagnetic wave, if at a point, at an instant, electric field is along +y axis and magnetic field is along +z axis, then the direction of propagation of the wave must be
1. +x axis
2. -x axis
3. -y axis
4. Both 1 & 2 are possible
A current flows in a wire of circular cross section with the free electrons travelling with a drift velocity v. If an equal current flows in a wire of the same material but of twice the radius, the new mean drift speed is
(1) (2) 4v (3) v (4) 2v
In a common emitter amplifier, the phase difference between input signal and output signal is:
1. zero
2.
3.
4.
In an NPN transistor amplifier, if 98% of electrons emitted from the emitter reach the collector, then the value of collector current for the base current of 40 A will be:
1. 1.96 mA
2. 1.92 mA
3. 1.96 A
4. 1.92 A
The Boolean expression for the output Y of the circuit is given by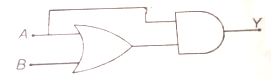
1. A
2. A + B
3. AB
4. A - B
A 2 capacitor is charged to a potential 100 V and a 4 capacitor is charged to a potential 50 V. If the capacitors are connected in parallel such that their similar plates are connected together, then the loss of energy due to parallel connection is
(1) 1.7 J (2) 1.7
(3) 1.7 (4) 1.7
| 1. | It may emit \(\alpha\text-\)particle. |
| 2. | It may emit \(\beta^{+}\) particle. |
| 3. | It may go for \(K\) capture. |
| 4. | All of the above are possible. |
In a nucleus, the mass number is related with its volume as
(1)
(2) V A
(3) V
(4)
In the nuclear reaction , if a is binding energy per nucleon for and b is binding energy per nucleon for then the energy released in the reaction is
(1) b-a (2) 2b-2a (3) 4b-2a (4) 4b-4a
In the figure, A is a circular coil of wire carrying an anticlockwise direction . C is an infinite long straight wire carrying a current perpendicular into the plane of paper through the centre of the coil. The force on C due to A is (R -radius)
1. 2.
3. 4. zero
A loop is kept in xy plane as shown. The direction of its dipole moment is
(1) Along the positive x-axis
(2) Along the positive z-axis
(3) Along the negative z-axis
(4) Along the negative x-axis
The time period of an electron in third excited state is x times the time period in its ground state in a Bohr atom, then the value of x must be
1. 27
2. 16
3. 64
4. 9
In a fission reaction a nucleus breaks into two nuclei in mass ratio 1 : 2. The de-Broglie wavelengths of the nuclei will be in the ratio of
(1) 1 : 2 (2) 1 : 1 (3) 2 : 1 (4) 4 : 1
The work function of a metal is 4.0 eV. When light of wavelength 4000 is made to fall on the metal in a photoelectric experiment, the value of stopping potential will be
(1) 0.9 V (2) 3.1 V (3) 4.0 V (4) zero
Which one of the following graphs best represents the variation of magnitude of stopping potential with frequency of incident light in a photoelectric experiment?
1.
2. 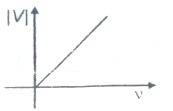
3. 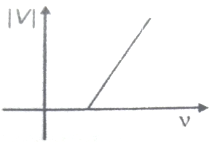
4.