To protect and improve the quality of our environment, government of India has passed Environment (Protection) Act in the year ______
1. 1974
2. 1981
3. 1986
4. 1987
Read the following statements and choose the correct option
Statement A : Electrostatic precipitator can remove over 99% particulate matter present in exhaust from a thermal power plant.
Statement B : According to CPCB particulate size PM2.5 or less in diameter are responsible for causing the greatest harm to human health.
1. Only satatement A is correct.
2. Only statement B is correct.
3. Both statements A and B are correct.
4. Both statements A and B are incorrect.
State True (T) or False (F) for the following statements and select the correct option
A. Motor vehicles equipped with catalytic converter should use unleaded petrol because lead in the
petrol inactivates the catalyst.
B. Recently government of India has instituted the Amrita Devi Bishonoi Wildlife Protection Award
for individuals from urban areas that have shown extraordinary courage and dedication in
protecting wildlife.
C. Reforestation may occur naturally in a deforested area.
A B C
1. T T F
2. T F T
3. T F F
4. F F T
Triangular age pyramid represents
1. Expanding population.
2. Declining population.
3. Mature population.
4. Both (a) and (c)
What do you mean by bioprospecting?
1. Biological analysis of living things to classify them?
2. Exploring molecular, genetic and species level diversity for product of economic importance.
3. Exploring forests to identify diversity present there.
4. It is branch of biology which deals with prospect of conservation.
Which of the following is not correct with respect of control of vehicular air pollution in India?
1. Use of CNG as fuel
2. Not phasing out of old vehicles as they have better technology
3. Use of unleaded petrol, low-sulphur petrol and diesel
4. Use of catalytic converter
Each tropic level has certain mass of living material at a particular time called
1. Standing crop
2. Standing state
3. GPP
4.NPP
Term ‘biodiversity’ was popularized by
1. Robert May
2. Edward wilson
3. Paul Ehrlich
4. C. Mobius
Choose the incorrect match regarding examples of recent extinctions.
1. Quagga - Africa
2. Steller’s Sea Cow - Russia
3. Dodo - Myanmar
4. Thylacine - Australia
Read the following statements and choose the correct option
Statement 1 : The success of mammals is largely due to their ability to maintain a constant body temperature.
Statement 2 : Majority of the animals and nearly all plants cannot maintain a constant internal environment.
1. Only statement 1 is correct.
2. Only statement 2 is correct.
3. Both statements are correct.
4. Both statements are incorrect.
Select the correct option regarding relative contribution of various greenhouse gases to total global warming.
Select the incorrect statement
1. Species diversity decreases as we move away from the equator towards the poles.
2. The IUCN red list (2004) documents the extinction of 784 species in the last 500 years.
3. Amazon rainforest is so huge that it is called the ‘heart of the planet’
4. Extinction of Steller’s sea cow and passenger pigeon were due to their overexploitation by humans.
Who coined the term ecosystem and defined it as the sum total of interactions between biotic and abiotic components?
1. C. Mobius
2. A.G. Tansley
3. Forbes
4. Friederich
Detritus is
1. Dead raw material for decomposition.
2. Partially decomposed organic matter.
3. Dead remains of animals & plants.
4. Both 1 and 3
In aquatic ecosystem, major channel for energy flow is
1. Grazing food chain.
2. Detritus food chain.
3. Auxillary food chain.
4. Parasitic food chain.
State True (T) or False (F) for the following statements and select the correct option
a. Mammals from colder climates generally have smaller ears and limbs to minimize the heat loss.
b. Experience of altitude sickness is due to high atmospheric pressure at high altitudes.
c. The size of a population for any species is not a static parameter.
d. Resources for growth for most of the animal population are infinite and become limiting sooner or later.
a b c d
1. T T T T
2. F T T F
3. F F F F
4. T F T F
If ‘N’ is the population density at time t, then its density at time ‘t+1’ will be
1.
2.
3.
4.
Choose the incorrect match regarding population interactions.
Species A Species B
1. Ammensalism – –
2. Parasitism + –
3. Commensalism + 0
4. Mutualism + +
Which of the following statements is/are correct?
(1) The entire sequence of communities that successively change in a given area is called sere.
(2) The natural reservoir of phosphorus is rock.
(3) Ecological pyramids do not accommodate food web.
1. Only statement (1) is correct.
2. Only statement (2) is correct.
3. All (1), (2) and (3) are correct.
4. All (1), (2) and (3) are incorrect.
In most ecosystems, all the pyramids of number, energy and biomass are upright. It indicates that
(1) Producers are more in number and biomass than the herbivores.
(2) Herbivores are less in number and biomass than the carmivores.
(3) Energy at a lower tropic level is always more than at a higher level.
Choose the correct option.
1. Only (1) is correct.
2. Only (2) is correct.
3. Both (1) and (3) are correct.
4. Both (2) and (3) are correct.
In which part of the atmosphere ‘good ozone’ is found?
1. Troposphere
2. Stratosphere
3. Ionosphere
4. Exosphere
During primary succession on rocks, pioneer species are
1. Annual grasses.
2. Perennial grasses.
3. Xerophytic shrubs.
4. Lichens.
Out of total cost of various ecosystem services, the soil formation accounts for about __A__, and contribution of other services like recreation and nutrient cycling, are less than __B__ each.
In the above statement for ‘A’ and ‘B’, which option is correct?
1. A – 6%, B – 10%
2. A – 50%, B – 10%
3. A – 10%, B – 50%
4. A – 10%, B – 6%
What is/are the correct explanation(s) for the species richness of the tropics?
1. Tropics had less evolutionary time for species diversification.
2. Tropics provide a relatively constant environment.
3. Tropics receive more solar energy which contributes to greater productivity.
4. Both (b) and (c) are correct.
Which one is not an effect of increase in the level of greenhouse gases?
1. CO2 fertilization effect.
2. EI Nino effect.
3. Snow blindness.
4. Warming of troposphere and cooling of stratosphere and thermosphere
In terrestrial ecosystem, a much larger fraction of energy flows through______
1. GFC
2. DFC
3. Parasitic food chain
4. Both (a) and (c)
Read the following statements and choose the correct option
Statement - 1 : Food webs provide stability to food chain.
Statement - 2 : Food web operates because of taste preference for particular food and unavailability of food.
1. Only statement (2) is correct.
2. Only statement (1) is correct.
3. Both statements are correct.
4. Both statements are incorrect
A person who is working as Disc Jockey (DJ) might suffer from?
1. Psychological and physiological disorder
2. Sleeplessness, increased heart beating, altered breathing pattern
3. Permanent lose of hearing ability in long run
4. All the above
Initially, how many biodiversity hot spots were identified globally?
1. 34
2. 25
3. 20
4. 30
National Forest Policy __A__ of India has recommended __B__ forest cover for the plains and __C__ for the hills. Fill the blanks with suitable A, B and C
A B C
1. 1988 33% 67%
2. 1988 30% 19.4%
3. 1989 30% 65%
4. 1987 19.4% 30%
‘Chipko Movement’ was originated in Himalayan region. At present this region is a part of ______
1. Uttar Pradesh
2. Uttarakhand
3. Himachal Pradesh
4. Jammu and Kashimir
In logistic growth pattern, the influence of environmental resistances over the biotic potential is denoted by
1. rN
2.
3.
4.
Read the statements given below and choose the correct option
Statement I : Decomposition is purely an anaerobic process.
Statement II : The rate of decomposition is controlled by chemical composition of detritus and climatic factors.
1. Only statement I is correct.
2. Only statement I is incorrect.
3. Both statements I and II are correct.
4. Both statements I and II are incorrect.
Biomagnification of DDT in aquatic food chain leads to high concentration of DDT in fish eating birds. It results in
| 1 | Disturbance in calcium metabolism in birds. |
| 2 | Thinning of egg shell and their premature breaking. |
| 3 | Decline in birds population |
1. (1) and (2) only
2. (2) and (3) only
3. (1) and (3) only
4. All (1), (2) and (3)
Which one is not a cause of biodiversity loss?
1. Alien species invasion.
2. Co-extinction.
3. Endemism.
4. Overexploitation.
Select the odd one with respect to strategies of biodiversity conservation
1. Biosphere reserves.
2. Sacred groves.
3. National parks.
4. Zoological parks.
Match the columns and select the correct option
| Column-I | Column-II | ||
| A. | Earth Summit | (i) | Johannesburg |
| B. | World summit on sustainable Development. | (ii) | Broadly utilitarian |
| C. | Biodiversity plays a major role in many ecosystem services. |
(iii) | Narrowly utilitarian |
| D. | Direct economic benefits from nature. | (iv) | Rio de Janerio |
1. A – (iv), B– (i), C– (ii), D– (iii)
2. A – (i), B– (iv), C– (iii), D– (ii)
3. A – (iv), B– (i), C– (iii), D– (ii)
4. A – (i), B– (iv), C– (ii), D– (iii)
Which one is not a key criteria for determining a hotspot?
| 1. | Very high levels of species richness. |
| 2. | High degree of those species which are confined to that region and not found anywhere else. |
| 3. | Degree of threat, which is measured in terms of habitat loss. |
| 4. | No habitat loss. |
The ‘niche’ of a species is meant for
1. Habitat and specific functions of a species
2. Specific place where an organism lives
3. Specific species function and its competitive power
4. Both (a) and (c)
The population of an insect species shows an explosive increase in numbers during rainy season followed by an eruptive fall at the end of the season. What does this show?
1. The food plants mature and die at the beginning of therainly season
2. Its population growth curve is of J-type
3. The population of its predators increases enormously
4. S-shaped or sigmoid growth of this insect
Study the four statement (1-4) given below and select the two correct ones out of them
(1) A lion eating a deer and sparrow feeding on grain are ecologically similar in being consumers
(2) Predator star fish Pisaster helps in maintaining species diversity of some invertebrates
(3) Predators ultimately lead to the extinction of prey species
(4) Production of chemicals such as nicotine, strychnine by the plants are metabolic disorders
The two correct statements are
1. (1) and (2)
2. (2) and (3)
3. (3) and (4)
4. (1) and (4)
Select incorrect statement
1. Organisms living in oceans, lakes and rivers do not face any water related problems
2. Productivity and distribution of plants is dependent on water
3. The levels of thermal tolerance of different species determine to a large extent their geographical distribution
4. Foraging, reproductive and migratory activities of some animals are dependent on seasonal variation in light
Predation, parasitism and commensalisms share a common characteristic i.e.,
1. Both the interacting species are benefited
2. Interacting species live closely together
3. One of the species is benefited while other is harmed
4. Both the species belong to same taxonomic group
Which of the following is incorrect with respect to competition?
1. Resources need not be limiting for competition to occur
2. Competitive species may evolve mechanism that promote their co-existance
3. Connell’s field experiment is an example of competitive release
4. Only closely related species can show compeitition
Parasites evolved special adaptations in accordance with their life styles. Choose odd one out with respect to these adaptations.
1. High reproductive capacity
2. Simple life cycle and complex morphological, anatomical features
3. Loss of unnecessary sense organs
4. Loss of digestive system
Identifying all the genes in the genome that are transcribed into RNA is called as:
1. Expressed Sequence Tag
2. Sequence Annotation
3. Inverse PCR
4. Retrotranspos
The last of 24 human chromosomes to be sequenced was:
1. Chromosome 1 and completed in 2003
2. Chromosome 1 and completed in 2006
3. Chromosome X and completed in 2003
4. Chromosome X and completed in 2006
Satellite DNA:
1. Form a small portion of the human genome
2. Code for proteins that are essential for survival
3. Code for proteins that are not essential for survival
4. Show high degree of polymorphism
The size of VNTR varies from:
1. 0.1 to 20 kb
2. 0.1 to 2.0 mb
3. 0.1 to 0.2 bp
4. 0.1 to 2.0 bp
For the multiplication of any alien piece of DNA in an organism it needs to be a part of a chromosome that has a specific:
1. Telomeric sequence
2. Multiple cloning site
3. Ori
4. Selectable marker
The first type II restriction endonuclease whose functioning depended on a specific DNA nucleotide sequence was:
1. EcoRI
2. HindII
3. SmaI
4. BamHI
Which of the following cannot be used as a vector in rDNA technology?
1. Plasmid
2. Phage DNA
3. Bacterium
4. YAC
The following palindrome is recognized by the restriction enzyme:
1. BamHI
2. EcoRI
3. HindII
4. PstI
Which of the following does not have the ability to replicate within bacterial cells independent of the control of chromosomal DNA?
1. Plastid
2. Bacteriophages
3. BAC
4. Plasmid
pBR322 does not contain the site for:
1. Pvu I
2. BamH I
3. Sma I
4. EcoR I
A method used only for transforming animal cells is:
1. Biolistics
2. Microinjection
3. Use of virus
4. Agrobacterium mediation
Lysozyme should be used when isolating DNA in a pure form from:
1. A bacterial cell
2. A fungal cell
3. A plant cell
4. An animal cell
If any protein encoding gene is expressed in a hetrologous host, the protein formed is called:
1. Recombinant protein
2. Native protein
3. Pro-protein
4. Exotic protein
Identify (a) and (b) bioreactors respectively in the given diagram: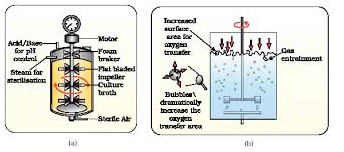
1. Simple stirred-tank and sparged stirred - tank
2. Sparged stirred - tank and simple stirred-tank
3. Airlift and tower
4. Tower and airlift
Why does Bt toxin not kill Bacillus thuringiensis?
1. The bacillus keeps proteins in inclusions
2. The toxin exists in the bacillus as a pro-toxin
3. The bacillus protects its genetic material by methylation
4. The bacillus has a tough cell wall of peptidoglycan
Cotton plants can be protected from corn borer by the proteins coded by:
1. cryIAc 2. cryIIAb
3. cryIAb 4. cryIAd
RNAi, as a mechanism of cellular defense, takes place in:
1. All bacteria
2. All unicellular organisms
3. All plants only
4. All eukaryotic organisms
How were nematode-specific genes introduced in tobacco plants?
1. Using page DNA
2. Using a retrovirus
3. By gene gun
4. Using Agrobacterium tumefaciens
The first recombinant therapeutic product approved for human use was:
1. Humulin
2. hGH
3. Factor VIII clotting factor
4. Erythropoietin
The first clinical gene therapy trial was given in 1990 to a 4 year girl with:
1. Alpha – 1 antitrypsin deficiency
2. Cystic fibrosis
3. Adenosine deaminase deficiency
4. Chronic myeloid leukemia
ELISA is based on the principle of:
1. Antibody opsonization
2. Antigen – antibody interaction
3. Radioactive immune assay
4. Creation of recombinant DNA
Over 95 % of all transgenic animals are:
1. Mice
2. Pigs
3. Sheep
4. Rabbits
The transgenic cow, Rosie, produced milk that:
1. Contained human beta lactalbumin
2. Interferons to treat viral infections
3. Alpha – 1 antirypsin
4. Is protein enriched
You want to transform the cells of sugarcane plant with the objective of introducing a disease resistant gene in sugarcane plants. The best method to achieve this goal would be:
1. The use of a gene gun
2. Microinjection
3. Agrobacterium tumefaciens mediation
4. Delivery of DNA by liposomes
Match each item in Column I with one in Column II and select the answer from the codes given below:
|
|
COLUMN I |
|
COLUMN II |
|
A |
First genetically modified crop |
a |
Antibiotic resistant tobacco plant |
|
B |
First genetically modified food |
b |
FlavrSavr |
|
C |
First genetically modified animal approved for food use |
c |
AquAdvantage Salmon |
|
D |
First genetically modified animal to be commercialized |
d |
GloFish |
Codes
A B C D
1. a b c d
2. a b d c
3. b a c d
4. b a d c
Recombinant therapeutic [used for treatment] products are available for all the following disorders except:
1. Hemophilia A
2. Pituitary dwarfism
3. Diabetes insipidus
4. Anemia due to renal failure
Electroporation is:
1.the process of separating charged molecules through a gel maintained in an electric field
2.the process of combining foreign DNA to an electrically charged vector molecule
3.the process of introducing DNA into cells by the application of high voltage pulses
4.the process of protoplast fusion by the application of high voltage pulses
Restriction endonucleases present in bacterial cells provide advantage to the cell because these enzymes:
1.can identify the mutated sequences on the chromosomal DNA and help in their repair
2.selectively bind to particular nucleotide sequences that may appear in viral DNA preventing its replication in the cell
3.can cut the plasmid DNA of the bacterial cell that allows them to recombine foreign DNA into their plasmid DNA
4.help the bacterium take up foreign DNA from their environment and combine it into their genome
Which of the following primers would allow copying of the single-stranded DNA sequence 5' ATGCCTAGGTC?
1. 5' ATGCC
2. 5' TACGG
3. 5' CTGGA
4. 5' GACCT
The "Southern Blot" technique refers to:
1.the detection of RNA fragments on membranes by specific radioactive antibodies.
2.the detection of DNA fragments on membranes by a radioactive DNA probe.
3.the detection of proteins on membranes using a radioactive DNA probe.
4.the detection of DNA fragments on membranes by specific radioactive antibodies.
The salient features of the human genome include all except:
1. About 1.4 million SNP locations
2. Functions known for less than 50% of the discovered genes
3. The actual number of genes and the initial estimates are remarkable similar
4. More than 98% of genome does not code for proteins
Fred Sanger designed a method of DNA sequencing based on the fact that when aadideoxy base is encountered the DNA synthesis:
1. commences
2. continues
3. stops
4. increases
Match each item in Column I with one in Column II and select the answer from the codes given below:
|
COLUMN I |
COLUMN II |
||
|
A |
Bioprospecting |
a |
the process of discovery and commercialization of new products based on biological resources |
|
B |
Biopiracy |
b |
the exploitative patenting of already widely used natural resources, such as plant varieties, by commercial entities |
|
C |
Bioremediation |
c |
a process that uses microorganisms or their enzymes to treat polluted sites for regaining their original condition |
|
D |
Biofortification |
d |
the process by which the nutritional quality of food crops is improved through agronomic practices, conventional plant breeding, or modern biotechnology |
Codes:
A B C D
1. a b c d
2. a b d c
3. b a c d
4. b a d c
Antisense technology:
1. selectively blocks expression of a gene.
2. combines genetic material from different species.
3. combines organelles and cells.
4. alters or transfers cells.
A direct target for gene therapy would include all the following cells except:
1. erythrocytes
2. muscle fibers
3. hepatocytes
4. vascular endothelium
In recombinant DNA experiments, a vector:
1. carries DNA into a new cell
2. links together newly joined fragments of DN
3. makes millions of copies of a specific segment of DNA
4. separates fragments of DNA by their length and electrical charges
Restriction Fragment Length Polymorphism (RFLP) is:
1.a variation in length of DNA fragments due to inherited differences in proteins produced
2.a variation in length of DNA fragments due to random differences in proteins produced
3.a variation in length of DNA fragments due to inherited differences in highly repetitive DNA
4.a variation in length of DNA fragments due to random differences in highly repetitive DNA
It is useful to use restriction enzymes in rDNA experiments that produce sticky ends in the resultant fragments because:
1. it allows a cell to recognize fragments produced by the enzyme
2. the single-stranded ends serve as starting points for DNA replication
3. the fragments will bond to other fragments with complementary single-stranded ends
4. only single-stranded DNA segments can code for proteins
Gene cloning occurs:
1. when a phage transfers bacterial DNA from one bacterium to another.
2. when a bacterium takes up DNA from the surrounding fluid.
3. when a bacterium carrying a recombinant plasmid reproduces, thus allowing for the production of multiple copies of the recombinant plasmid.
4. when DNA is produced from an RNA template.
Restriction enzymes do not act on the DNA of the Host cell in which they are produced because
1. Host DNA is packed into chromosomes
2. Restriction enzymes are ineffective on host DNA
3. Host DNA does not have the restriction site for the Restriction enzymes.
4. Restriction site of host DNA is methylated.
A protein is not expressed properly in a diseased tissue. To find out whether the defect is at the level of translation or post translational modifications which techniques would you use ?
1. Southern blotting and South Western blotting
2. Northern blotting and western blotting
3. Western blotting and Eastern blotting
4. Southern and Northern blotting
Which among the following is a cloning vector?
1. EcoRI
2. BamHI
3. pUC18
4. Sal I
Which of the following is a recombinant protein?
1. Beta Galactosidase in E.coli
2. Insulin in humans
3. Somatotropin in humans
4. Interferon in E. coli
The study of all the proteins encoded by the genome of an organism is called
1. Proteome
2. Proteomics
3. Translation studies
4. Genomics
Look at the given hypothetical vector-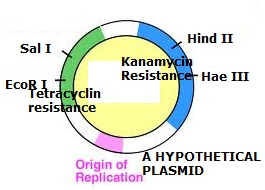
Suppose we cut the desired gene as well as the plasmid with the help of the Restriction enzyme EcoR I and ligate them. What would be true-
1. Kanamycin resistance will select the transformants from non transformants while tetracycline resistance will select recombinant transformants from non-recombinant transformants.
2. Tetracyclin resistance will select the transformants from non transformants while kanamycin resistance will select recombinant transformants from non-recombinant transformants.
3. Both will select only transformants.
4. Both will select only recombinants.
Among the following ions, which one has the highest paramagnetism ?
1.
2.
3.
4.
In the complex ion has five d-electrons and L is a weak field ligand. According to crystal field theory, the magnetic properties of the complex ion correspond to how many unpaired electrons?
1. 0
2. 5
3. 2
4. 3
Diethylene triamine is :
1. Chelating agent
2. Polydentate ligand
3. Tridentate ligand
4. All
In the coordination compound (en=ethylenediamine), the coordination number and oxidation number of the central atom are, respectively :
1. 4, +3
2. 6, +2
3. 4, +2
4. 6, +3
Which of the following is p complex:
1. Trimethyl aluminium
2. Ferrocene
3. Diethyl zinc
4. Nickel carbonyl
The number of d-electrons of Cr(Z=24) in
1. 2
2. 3
3. 4
4. 5
The geometries of Ni(CO)₄ and Ni(PPh₃)₂Cl₂ are, respectively:
1. Both square planar
2. Tetrahedral and square planar
3. Both tetrahedral
4. Square planar and tetrahedral
The complex ion which has no ‘d’ electrons in the central metal atom is
1.
2.
3.
4.
The compound(s) that exhibit(s) geometrical isomerism is(are)
1. [Pt(en)Cl2]
2. [Pt(en)2]Cl2
3. [Pt(en)2Cl2]Cl2
4. [Pt(NH3)2Cl2]
A. 1,2
B. 2,4
C. 1,4
D. 2,3
The spin only magnetic moment value (in Bohr magneton units) of Cr(CO)6 is -
1. 0
2. 2.84
3. 4.90
4. 5.92
The ionization isomer of [Cr(H2O)4 Cl(NO2)] Cl is -
1. [Cr(H2O)4 (O2N)] Cl2
2. [Cr(H2O)4 Cl2](NO2)
3. [Cr(H2O)4 Cl(ONO)]Cl
4. [Cr(H2O)4 Cl2(NO2)]. H2O
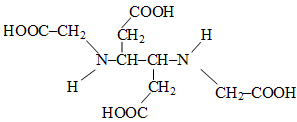
The correct structure of ethylenediaminetetraacetic acid (EDTA) is -
1. 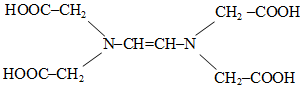
2. 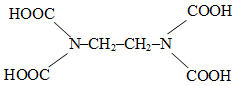
3. 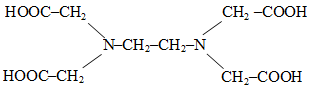
4. 
The complex showing a spin-only magnetic moment of 2.82 B.M. is-
1. Ni(CO)4
2. [NiCl4]2–
3. Ni((PPh3)4
4. [Ni(CN)4]2–
As per IUPAC nomenclature, the name of the complex [Co(H2O)4(NH3)2]Cl3 is -
1. Tetraaquadiaminecobalt (III) chloride
2. Tetraaquadiamminecobalt (III) chloride
3. Diaminetetraaquacobalt (III) chloride
4. Diamminetetraaquacobalt (III) chloride
NiCl2{P(C2H5)2(C6H5)}2 exhibits temperature dependent magnetic behaviour (paramagnetic / diamagnetic). The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are respectively
1. tetrahedral and tetrahedral
2. square planar and square planar
3. tetrahedral and square planar
4. square planar and tetrahedral
Which of the following observations/statements is/are correct ?
1. Anhydrous becomes blue in aqueous medium due to the complex formation
2. dissolves in KCN giving an orange-red solution
3. can be precipitated by adding
4. None of the above
Arrange the following in increasing order of their magnetic moment
1.
2.
3.
4.
Manganese show oxidation state from + 2 to + 7. The most oxidizing state known in aqueous solution is
1. + 7
2. + 4
3. + 3
4. + 2
When conc. is added to , explosion take place and the product formed is-
1.
2.
3.
4.
When
1.
2.
3.
4. All of these
Arrange the hydrides of group 15 in the order of increasing boiling points -
1.
2.
3.
4.
Red phosphorus is chemically unreactive because -
1. It does not contain P — P bonds
2. It does not contain tetrahedral molecules
3. It does not catch fire in air even upto 400º C
4. It has a polymeric structure.
The arrangement of oxygen atoms around phosphorus atoms in P4O10 is -
1. Pyramidal
2. Octahedral
3. Square planar
4. Tetrahedral
The highest bond strength is shown by
1. O – O bond
2. S – S bond
3. Se – Se bond
4. Te – Te bond
Freezing point of is -
1. – 183ºC
2. – 229ºC
3. – 195.8ºC
4. – 186ºC
Ozone acts as
1. Oxidising agent
2. reducing agent
3. bleaching agent
4. all
Which of the following is / are ionic in character –
.
.
.
. SnCl4
1. . ,
2.,
3.,SnCl4
4. SnCl4,
Pick out the incorrect statement regarding halogens –
1. Chlorine is hydrolysed by water to form hydrochloric acid and hypochlorous acid
2. Bromine and iodine react with NaOH solution to form halide and halite ion
3. Chlorine reacts with cold dilute NaOH solution to give sodium chloride and sodium chlorite
4. Iodine forms a deep blue colour with starch solution
The halide which does not give a precipitate with is –
1. F¯
2. Cl¯
3. Br¯
4. I¯
Bromine is prepared in the laboratory –
1. by heating KBr with Conc. H2SO4
2. by heating KBr, MnO2 with Conc. H2SO4
3. by heating KBr with HCl
4. by passing I2 vapours through KBr solution
Reducing agent used for reduction of copper oxide in blast furnace is
1. coke
2. lime stone
3. aluminium
4. no reducing agent is used
Product obtained after Bessemerisation is called as ............ because
1. concentrated copper ; copper percentage is high
2. copper matte ; of its appearance
3. blister copper ; of its appearance
4. ultra pure copper ; 100 percent copper
Considering 100% pure, which of the following has maximum percentage of Pb ?
1. galena
2. anglesite
3. cerussite
4. lanarkite
Function of H2SiF6 in electrolyte during electrolytic refining is
1. to decrease the operating temperature
2. to increase the conductivity of the bath
3. to separate impurities easily
4. to maintain electrical neutrality
Gold is extracted by making soluble cyanide complex. The cyanide complex is
1.
2.
3.
4.
Galvanisation means :
1. decomposition of Zn on Fe
2. decomposition of Al on Fe
3. decomposition of Sn on Fe
4. decomposition of Cu on Fe
If NaOH is added to an aqueous solution of Zn+2, a white precipitate appears and on adding excess of NaOH the precipitate dissolves. In the solution Zn exists in the:
1. Cationic part
2. Anionic part
3. In both the parts
4. There is no Zn is solution
Generally transition metals act as catalyst because of –
1. free valencies
2. large surface area
3. unpaired d- electrons
4. all of these
Zn and Hg belong to the same group, they differ in many of their properties. The property that is shared by both is –
1. they form oxide readily
2. they react with steam readily
3. they react with dil. H2SO4
4. they react with hot NaOH
Match the compounds of column X with oxidation state of column Y–
Column X Column Y
I. [Cr(H2O)6]Cl3 5
II. CrO5 4
III. K3CrO8 6
IV. (NH4)3 CrO4 3
I II III IV
1. 3 6 5 4
2. 3 4 5 6
3. 4 5 6 3
4. 6 5 4 3
A pale green crystalline salt of M dissolves freely in water. It gives a brown precipitate on addition of aqueous NaOH. The metal salt solution also gives a black precipitate on bubbling H2S in acid medium. An aqueous solution of the metal salt decolourises the pink colour of the permanganate solution. The metal in the metal salt solution is–
1. Cu
2. Al
3. Pb
4. Fe
For Ni and Pt different I.P. values are given below–
(IP)1 + (IP)2 (IP)3 + (IP)4
Ni 2.49 8.80
Pt 2.60 6.70
Hence :
1. nickel (II) compounds tend to be thermodynamically more stable than platinum (II)
2. platinum (IV) compounds tend to be more stable than nickel (IV)
3. both correct
4. None is correct
Which of the following reactions is not correct?
1. 2Na2CrO4 + H+ Na2Cr2O7 + 2Na+ + H2O
2. 2MnO2 + 4KOH + O2 4KMnO4 + 2H2O
3. Mn +8H+ +5Fe2+ 5Fe3+ + Mn2+ + 4H2O
4. 2Mn + 5C2+ 16H+ 2Mn2+ + 10CO2 + 8H2O
Which of the following compounds exhibit the same colour in the aqueous solution ?
1. VOCl2 & FeCl2
2. FeCl2 & CuCl2
3. MnCl2 & FeCl2
4. VOCl2 & CuCl2
A solution of a metal ion when treated with KI gives a red precipitate which dissolves in excess KI o give a colourless solution. Moreover, the solution of metal ion on treatment with a solution of cobalt(II) thiocyanate gives rise to a deep blue crystalline precipitate. The metal ion is
1. Pb2+
2. Hg2+
3. Cu2+
4. Co2+
The electron in a hydrogen atom make a transition from an excited state to the ground state. Which of the following statements is true?
1. Its kinetic energy increases and its potential and total energy decrease.
2. Its kinetic energy decreases, potential energy increases and its total energy remains the same.
3. Its kinetic and total energies decreases and its potential energy increases.
4. its kinetic, potential and total energies decreases.
A photoelectric cell is illuminated by a small bright source of light placed at 1m. If the same source of light is placed 2m away, the electrons emitted by the cathode
1. each carries one quarter of its previous momentum.
2. each carries one quarter of its previous energy.
3. are half the previous number.
4. are one quarter of the previous number.
The work function of a substance is 4.0 eV. The longest wavelength of light that can cause photoelectron emission from this substance is approximately
1. 540 nm
2. 400 nm
3. 310 nm
4. 220nm
The de-Broglie wavelength of a particle moving with a velocity 2.25 × 108 m/s is equal to the wavelength of photon. The ratio of kinetic energy of the particle to the energy of the photon is (velocity of light is 3 × 108 m/s)
1. 1/8
2. 3/8
3. 5/8
4. 7/8
In a photoelectric experiment with the light of wavelength , the fastest electron has speed v. If the exciting wavelength is changed to , the speed of the fastest emitted electron will become
1.
2.
3. less than
4. greater than
An element \(\mathrm{X}\) decays, first by positron emission, and then two \(\alpha\text-\)particles are emitted in successive radioactive decay. If the product nuclei have a mass number \(229\) and atomic number \(89\), the mass number and the atomic number of element \(\mathrm{X}\) are:
1. \(237,~93\)
2. \(237,~94\)
3. \(221,~84\)
4. \(237,~92\)
90% of a radioactive sample is left undecayed after time t has elapsed. What percentage of the initial sample will decay in a total time 2t?
1. 20%
2. 19%
3. 40%
4. 38%
A radioactive element X converts into another stable element Y. Half life of X is 2 hrs. Initially only X is present After time t, the ratio of atoms of X and Y is found to be 1 : 4, then t in hours is:
1. 2
2. 4
3. between 4 and 6
4. 6
An electron in a hydrogen atom makes a transition from the first excited state to the ground state. The equivalent current due to circulating electron:
1. increases 2 times
2. increases 4 times
3. increases 8 times
4. remains the same
The maximum velocity of an electron emitted by light of wavelength incident on the surface of a metal of work function , is
1.
2.
3.
4.
When a point source of monochromatic light is at a distance of 0.2 m from a photoelectric cell, the cut-off voltage and the saturation current are 0.6 volt and 18 mA respectively. If the same source is placed 0.6 m away from the photoelectric cell, then
1. The stopping potential will be 0.2 V
2. The stopping potential will be 0.6 V
3. The saturation current will be 6 mA
4. The saturation current will be 18 mA
Work function of lithium and copper are respectively 2.3 eV and 4.0 eV. Which one of the metal will be useful for the photoelectric cell working with visible light ? (h = 6.6 10–34 J-s, c = 3 108 m/s)
1. Lithium
2. Copper
3. Both
4. None of these
As per Bohr model, the minimum energy (in eV) required to remove an electron from the ground state of doubly ionized Li atom (Z = 3) is:
1. 1.51
2. 13.6
3. 40.8
4. 122.4.
A hydrogen atom and a Li++ ion are both in the second excited state. If and are their respective electronic angular momenta, and EH and ELi their respective energies, then:
1. and |EH| >|ELi|
2. and |EH| < |ELi|
3. and |EH| >|ELi|
4. and |EH| < |ELi|
The maximum kinetic energy of photoelectrons emitted from a surface when photons of energy 6eV fall on it is 2eV. The stopping potential in volts is:
1. 2
2. 4
3. 6
4. 10
In a cathode ray tube, the distance between the cathode and the anode is 0.5 m and the potential difference is 50 kV. If an electron starts from rest from the cathode, then it will strike the anode with a velocity :
1. 2.66 × 108 m/s
2. 1.33 × 108 m/s
3. 0.66 × 108 m/s
4. 0.33 × 108 m/s
Two charged particles having charge Q and –Q, and masses m and 4m respectively enters in uniform magnetic field B at an angle with magnetic field from same point with speed v. The displacement from starting point where they will meet again, is
(1) (2)
(3) (4)
| 1. | \(\dfrac{(Z - 13)}{\left(A - Z - 23\right)}\) | 2. | \(\dfrac{\left(Z - 18\right)}{\left(A - 36\right)}\) |
| 3. | \(\dfrac{\left(Z - 13\right)}{\left(A - 36\right)}\) | 4. | \(\dfrac{\left(Z - 13\right)}{\left(A - Z - 13\right)}\) |
| 1. | \(1.5\times 10^{17}\) | 2. | \(3\times 10^{19}\) |
| 3. | \(1.5\times 10^{25}\) | 4. | \(3\times 10^{25}\) |
In a mean life of a radioactive sample:
1. about 1/3 of substance disintegrates
2. about 2/3 of the substance disintegrates
3. about 90% of the substance disintegrates
4. almost all the substance disintegrates.
Two radioactive materials X1 and X2 have decay constants 10 and respectively. If initially they have the same number of nuclei, then the ratio of the number of nuclei of X1 to that of X2 will be 1/e after a time
1.
2.
3.
4.
The half-life of a radioactive substance is 3.6 days. How much of 20 mg of that radioactive substance will remain after 40 days?
1. 2.68 103 mg
2. 4.31 10–2 mg
3. 6.20 10–3 mg
4. 9.76 10–3 mg
Highly energetic electrons are bombarded on a target of an element containing 30 neutrons. The ratio of radii of nucleus to that of Helium nucleus is . The atomic number of nucleus will be
1. 25
2. 26
3. 56
4. 30
The zener breakdown will occur if:
| 1. | the impurity level is low. |
| 2. | the impurity level is high. |
| 3. | the impurity is less on the \(\mathrm{n\text-}\)side. |
| 4. | the impurity is less on the \(\mathrm{p\text-}\)side. |
The logic behind the 'NOR' gate is that it gives:
| 1. | High output when both the inputs are low. |
| 2. | Low output when both the inputs are low. |
| 3. | High output when both the inputs are high. |
| 4. | None of these |
In a PN-junction diode
1. The current in the reverse biased condition is generally very small
2. The current in the reverse biased condition is small but the forward biased current is independent of the bias voltage
3. The reverse biased current is strongly dependent on the applied bias voltage
4. The forward biased current is very small in comparison to reverse biased current
Two PN-junctions can be connected in series by three different methods as shown in the figure. If the potential difference in the junctions is the same, then the correct connections will be
1. In the circuit (1) and (2)
2. In the circuit (2) and (3)
3. In the circuit (1) and (3)
4. Only in the circuit (1)
Which is reverse-biased diode ?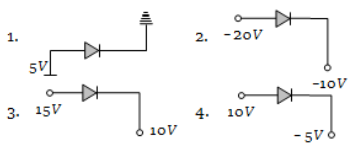
In a transistor circuit shown here, the base current is 35 A and the potential difference across emitter-base junction is 4.5 V. The value of the resistor Rb is:
1. 128.5 k
2. 257 k
3. 380.05 k
4. None of these
When NPN transistor is used as an amplifier
1. Holes move from emitter to base
2. Electrons move from emitter to collector
3. Electrons moves from base to emitter
4. Holes moves from base to emitter
In a transistor, a change of 8.0 mA in the emitter current produces a change of 7.8 mA in the collector current. What change in the base current is necessary to produce the same change in the collector current?
1. 50 A
2. 100 A
3. 150 A
4. 200 A
The emitter-base junction of a transistor is …… biased while the collector-base junction is ……. biased
1. Reverse, forward
2. Reverse, reverse
3. Forward, forward
4. Forward, reverse
Consider an NPN transistor amplifier in common-emitter configuration. The current gain of the transistor is 100. If the collector current changes by 1 mA, what will be the change in emitter current
1. 1.1 mA
2. 1.01 mA
3. 0.01 mA
4. 10 mA
An NPN transistor conducts when:
| 1. | both the collector and the emitter are positive with respect to the base. |
| 2. | the collector is positive and the emitter is negative with respect to the base. |
| 3. | the collector is positive and the emitter is at same potential as the base. |
| 4. | both the collector and the emitter are negative with respect to the base. |
In the case of constants and of a transistor
1.
2.
3.
4.
For a transistor, the current amplification factor is 0.8. The transistor is connected in common emitter configuration. The change in the collector current when the base current changes by 6 mA is
1. 6 mA
2. 4.8 mA
3. 24 mA
4. 8 mA
A \(2\) V battery is connected across the points \(A\) and \(B\) as shown in the figure given below. Assuming that the resistance of each diode is zero in forward bias and infinity in reverse bias, the current supplied by the battery when its positive terminal is connected to \(A\) is:
| 1. | \(0.2\) A | 2. | \(0.4\) A |
| 3. | zero | 4. | \(0.1\) A |
The diode used in the circuit shown in the figure has a constant voltage drop of 0.5 V at all currents and a maximum power rating of 100 milliwatts. What should be the value of the resistor R, connected in series with the diode for obtaining maximum current
1. 1.5
2. 5
3. 6.67
4. 200
In the circuit given below, if \(V(t)\) is the sinusoidal voltage source, then the voltage drop \(V_{AB}(t)\) across the resistance \(R\):
| 1. | is half-wave rectified. |
| 2. | is full-wave rectified. |
| 3. | has the same peak value in the positive and negative half-cycles. |
| 4. | has different peak values during the positive and negative half-cycles. |
An electron and a proton are separated by a large distance. The electron starts approaching the proton with energy 2eV. The proton captures the electron and forms a hydrogen atom in first excited state. The resulting photon is incident on a photosensitive metal of threshold wavelength 4600Å. The maximum K.E. of the emitted photoelectron is (Take hc = 12420 eV Å)
1. 2.4 eV
2. 2.7 eV
3. 2.9 eV
4. 5.4 eV
In the Boolean algebra , .A equals to
1.
2. A
3.
4. A+B
In a given circuit as shown the two input waveforms \(A\) and \(B\) are applied simultaneously. The resultant waveform \(Y\) is:

| 1. |  |
2. |  |
| 3. |  |
4. |  |