For which of the following organisms there is no natural death ?
1. Bacteria reproducing by sporulation fission
2. Yeast reproducing by budding
3. Unicellular organisms reproducing by spores
4. Unicellular organisms reproducing by binary
Vegetative reproduction is also a type of asexual reproduction." Which of the following statements justify this?
1. Involvement of one parent
2. Gametes are not involved
3. Does not involve meiosis
4. More than one option is correct
In algae and fungi homothallic term is used to represent
1. Dioecious condition
2. Unisexual condition
3. Bisexual condition
4. More than one option is correct
Read the following statement carefully:
"Further development of zygote depends on the type of life cycle the organism has and the environment it is exposed to."
Identify the correctly matched pair with respect to the above statement
|
1. Thick walled zygote |
Haplontic life cycle |
|
2. Zygote forms new generation, by mitosis, represented by one cell |
Haplodiplontic life cycle |
|
3. Zygote undergoes meiosis to form haploid generation |
Diplontic life cycle |
|
4. Zygote forms multicellular diploid generation |
Haplontic life cycle |
Choose the correct option from following statements
I. During ebryogenesis, zygote undergoes mitotic cell division
II. In organism with diplontic life cycle, zygote divides by meiotic cell division
III. The pericarp (fruit wall) develop from integument of ovule, after fertilization
IV. In brinjal, sepals remained attached to fruit even after fertilization
1. I,II are incorrect but III,IV are correct
2. III, IV are incorrect but II,III are correct
3. I,IV are incorrect but II,III are correct
4. II,III are incorrect but I,IV are correct
Entry of pollen tube into embryo sac is under ___guidance
1. Chemotropic
2. Chemotactic
3. Phototropic
4. Thigmotropic
Double fertilization was discovered by Nawaschin and Guignard in
1. Lilium and Allium
2. Lilium and Fritillaria
3. Zea mays and Mangifera
4. Nigella and Fritillaria
Why is vivipary an undesirable character for annual crop plants?
1. It reduces the vigour of the plant
2. It adverselt affects the fertility of the plant
3. The seeds exhibit long dormacy
4. The seeds cannot be stored under normal conditions for the next season
The pollen grain represents
1. Male gamete
2. Male gametophyte
3. Microsporophyll
4. Microsporangium
Choose the correct option with respect to the function of the germ pore.
1. It allows growth of pollen tube
2. It allows water absorption in seed
3. It helps dehiscence of pollen grain
4. More than one option is correct
The number of mitotic generations required to form a mature embryo sac from megaspore in most of the flowering plants is
1. One
2. Two
3. Three
4. Four
Hydrophily is limited to 30 genera which are mostly
1. Gymnosperms
2. Monocots
3. Dicots
4. More than one option is correct
Pollen pistil interaction is
1. Chemically mediated process
2. Dynamic process
3. Genetically controlled process
4. More than one option is correct
In the given diagram the gas X can be:
1. Hydrogen sulfide
2. Carbon monoxide
3. Ammonia
4. Oxygen
Choose the correct option from the following
I. Dehydration and dormancy of mature seeds are crucial for seed storage
II. Seed of Lupinus articus is the oldest one which germinated after 2000 year
III. Orchid seed is one of largest seed in plant kingdom
IV. Seeds are parasitic plants Orobanche and Striga are tiny seeds
1. I,II are correct but III,IV are incorrect
2. I,IV are correct but II,III are incorrect
3. III,IV are correct but I,II are incorrect
4. II,III are correct but I,IV are incorrect
A dioecious flowering plant prevents both
1. Autogamy and xenogamy
2. Autogamy and geitonogamy
3. Geitonogamy and xenogamy
4. Cleistogamy and xenogamy
The ovule of an angiosperm is technically equivalent to
1. Megasporangium
2. Megasporophyll
3. Megaspore mother cell
4. Megaspore
Examine the figure given below and select the right option giving all the four parts a,b,c and d. Correctly identify
| a | b | c | d | |
| 1. | Endo- thecium |
Tapetum | Micro- spore mother cell |
Middle layer |
| 2. | Tapetum | Endo- thecium |
Micro- spore mother cell |
Middle layer |
| 3. | Endo- thecium |
Middle layer |
Tapetum | Micro- spore mother cell |
| 4. | Endo- thecium |
Micro- spore mother cell |
Middle layer |
Tapetum |
Nucellar polyembryony is reported in species of
1. Brassica
2. Citrus
3. Gossypium
4. Triticum
What is common between vegetative reproduction and Apomixis?
1. Both occur round the year
2. Both produces progeny identical to the parent
3. Both are applicable to only dicot plants
4. Both bypass the flowering phase
What would be the number of chromosomes of the aleurone cells of a plant with 42 chromosomes in its root tip cells?
1. 21
2. 42
3. 63
4. 84
In case of a couple where the male is having a very low sperm count, which technique will be suitable for fertilisation?
1. Intrauterine transfer
2. Gamete intracytoplasmic fallopian transfer
3. Artificial Insemination
4. Intracytoplasmic sperm injection
In lactational amenorrhoea, there is no ovulation or menstruation during the period of intense lactation following parturition, due to high level of prolactin, which?
| 1. | Inhibits the release of gonadotropins |
| 2. | Inhibits the release of estrogen and progesterone |
| 3. | Stimulate the release of FSH and LH |
| 4. | Stimulates the release of estrogen and progesterone |
What is the function of copper ions in copper releasing IUDs?
| 1. | They increase phagocytosis of sperm within the uterus |
| 2. | They suppress sperm motility and the fertilising capacity of sperms |
| 3. | They make the uterus unsuitable for implantation |
| 4. | They inhibit ovulation |
Which of the following STD and its causative agent is not correctlty matched?
All the following statements about ZIFT are correct, but one is wrong. Which one is wrong?
1. It is zygote intra fallopian transfer
2. Zygote is transferred into the fallopian tube about after IVF
3. Early embryos upto 8 blastomeres can also be transferred into the fallopian tubes
4. Embryos with more than 8 blastomeres are also transferred into the fallopian tubes
In the human female, menstruation can be deferred by the administration of
1. FSH only
2. LH only
3. Combination of FSH and LH
4. Combination of estrogen and progesterone
In human, cleavage divisions are
1. Slow and synchronous
2. Fast and synchronous
3. Slow and asynchronous
4. Fast and asynchronous
A : In morula stage the cells divide without any increase in size without any increase in size
R : Zone pellucida remains intact till cleavage is completed
The extra embryonic membranes of the mammalian embryo are derived from
1. Trophoblast
2. Inner cell mass
3. Formative cells
4. Follicle cells
In the 28 day human ovarian cycle, the duration of luteal phase is approximate?
1. 14 days
2. 28 days
3. 30 days
4. 5 days
A change in the amount of yolk and its distribution in the egg will affect :
1. Pattern of cleavage
2. Number of blastomeres produced
3. Fertilization
4. Formation of zygote
Capacitation refers to changes in the
1. Sperm after fertilization
2. Sperm before fertilization
3. Ovum before fertilization
4. Ovum after fertilization
In which type of placenta minimum number of barrier is/are present between foetal and maternal blood?
1. Syndesmochorial
2. Haemochorial
3. Haemoendothelial
4. Endotheliochorial
Drugs such as Thalidomide taken by woman in first trimester of pregnancy cause all the following malformations in the developing embryo except
1. Phocomelia
2. Amelia
3. Heart disorder
4. Placentitis
In the fertile human female, approximately on which day of the menstrual cycle (32 days) does ovulation take place?
1. Day 18
2. Day 14
3. Day 1
4. Day 8
Which of the following groups of hormones produced in women only during pregnancy ?
1. hCG, hPL, relaxin
2. Estrogen, progesterone, hCG
3. Cortisol, prolactin, thyroxine
4. Prolactin. progesterone, hCG
The stem cells which have potency to give rise to all tissues and organs are formed from
1. Trophoblast
2. Umbilical cord
3. Inner cell mass
4. Placenta
Identify the hormones that are secreted in large amount prior to ovulation :
| A. | LH |
| B. | FSH |
| C. | Estrogen |
| D. | Progesterone |
1. A only
2. A & B only
3. A,B & C only
4. A,B,C & D
Which centriole is spermatozoa is required for first cleavage ?
1. Proximal centriole
2. Distal centriole
3. Ring centriole
4. Posterior centriole
Primary sex organs differ from the secondary sex organs in all the following, except
1. They produce gametes
2. They secrete sex hormones
3. They are concerned with the conduction of gametes
4. Testes in male and ovaries in female are the examples of primary sex organs
Which one of the following statements is incorrect about menstrual cycle ?
1. The first menstruation begins at puberty and is called menarche
2. Lack of menstruation may also occur due to some underlying factors like stress, poor health
3. Corpus luteum secretes large amounts of progesterone which is essential for maintenance of endometrium
4. In absence of fertilisation, corpus luteum degenerates in luteal phase and new follicles start developing immediately
Phase of menstrual cycle in human that lasts for 7-8 days is
1. Follicular phase
2. Ovulatory phase
3. Luteal phase
4. Menstruation
A change in ovum after penetration of sperm is
1. Formation of first polar body
2. Second meiosis starts
3. First meiosis
4. Formation of second polar body
Cryptochidism is
1. Non-development of testes
2. Nondescent of testes into scrotum
3. Removal of scrotum
4. Breaking connection of vas deferens
Scrotal sacs of man are connected with the abdominal cavity by
1. Inguinal canal
2. Haversian cana;
3. Spermatic canal
4. Rete testis
Which one of the following is important as one of the first line of defence against inhaled and ingested pathogen ?
1. IgA
2. IgG
3. IgM
4. IgD
A metastatic cancerous tumour is termed 'sarcoma' if the disorder is in
1. Fibroblasts
2. Circulatory system
3. Immune system
4. Epithelial cells
A person suffering from a disease caused by Plasmodium, experiences chill and fever at the time when?
| 1. | The sporozites released from RBCs are being rapidly killed and broken down inside spleen |
| 2. | The trophozites reach maximum growth and give out certain toxins |
| 3. | The parasite after its rapid multiplication inside RBCs ruptures them, releasing the stage to enter fresh RBCs |
| 4. | The microgametocytes and megagametocytes are being destroyed by the WBCs |
Alzheimer disesase in humans is associated with the deficiency of
1. Glutamic acid
2. Acetylcholine
3. Gamma aminobutyric acid (GABA)
4. Dopamine
Match the disease in Column l with the appropriate items (pathogen/prevention/treatment) in Column ll
Column l Column ll
a. Amoebiasis (i) Treponema palladium
b. Diphtheria (ii) Use only sterilized food and water
c. Cholera (iii) DPT Vaccine
d. Syphilis (iv) Use oral rehydration therapy
1. a(ii), b(iii), c(iv), d(i)
2. a(i), b(ii), c(iii), d(iv)
3. a(ii), b(iv), c(i), d(iii)
4. a(ii), b(i), c(iii), d(iv)
A person who met with a road accident is likely to develop tetanus, can be immunised by administering
1. Weakened germs
2. Dead germs
3. Preformed antibodies
4. Wide spectrum antibiotics
Antivenom injection contains performed antibiotics while polio drops that are administered into the body contain
1. Attenuated pathogens
2. Activated pathogens
3. Harvested pathogens
4. Gamma pathogens
MALT constitutes about ______ percent of the lymphoid tissue in human body
1. 50%
2. 20%
3. 70 %
4. 10%
Acid in stomach, saliva in the mouth, tears from eyes, all prevent microbial growth belong to which of the following barriers?
1. Physical barrier
2. Physiological barrier
3. Cellular barrier
4. Cytokine barrier
Asbestos causes cancer of
1. Liver
2. Lungs
3. Lungs and urinary bladder
4. Urinary bladder
Different species of Mycobacterium cause
1. Syphilis and Diphtheria
2. Whooping cough and leprosy
3. Tuberculosis and leprosy
4. Syphilis and gonnorhoea
Which of the following cancer is opportunistic disease associated with HIV ?
1. Cancer of cervix
2. Liver cancer
3. Pancreatic cancer
4. Kaposi's sarcoma
Which of the following is not an autoimmune disorder ?
1. Myasthenia gravis
2. Rheumatoid arthritis
3. Hashimoto's disease
4. Adam's-Stoke syndrome
Which of the following enhances or induces fusion of protoplasms ?
(1) IAA and gibberellins
(2) Sodium chloride and potassium chloride
(3) Polyethylene glycol and sodium nitrate
(4) IAA and kinetin
Which one of the following is a case of wrong matching ?
(1) Micropropagation-In vitro production of plants in large numbers
(2) Callus-Unorganised mass of cells produced in tissue culture
(3) Somatic hybridization-Fusion of two diverse cells
(4) Vector DNA-site for t-RNA synthesis
Consider the following four statements (a-d) and select the option which includes all the correct ones only
(a) Single Spirulina can produce large quantities of food rich in protein, minerals, vitamins etc.
(b) Body weight-wise the micro-organism Methylophilus methylotrophus may be able to produce several times more proteins than the cows per day
(c) Common button mushrooms are a very rich source of vitamin C
(d) A rice variety has been developed which is very rich in calcium
(1) Statements (c),(d)
(2) Statements (a),(c) & (d)
(3) Statements (b),(c) & (d)
(4) Statements (a), (b)
Consider the following four measures (a-d) that could be taken to successfully grow chick-pea in an area where bacterial blight disease is common
(a) Spray with Bordeaux mixture
(b) Control of insect vector of the disease pathogen
(c) Use of only disease-free seeds
(d) use of varieties resistant to the disease
Which two above measures can control the disease ?
(1) (a) and (d)
(2) (b) and (c)
(3) (a) and (b)
(4) (c) and (d)
Triticale, the first man-made cereal crop has been obtained by crossing wheat with
(1) Rye
(2) Pearl millet
(3) Sugarcane
(4) Barley
Three crops that contribute maximum to global food grain production are
(1) Wheat , rice and maize
(2) Wheat , maize and sorghum
(3) Rice, maize and sorghum
(4) Wheat, rice and barley
Which of the following is wrongly matched in the given table ?
| Microbe | Product | Application | |
| 1. |
Clostridium |
Lipase |
Removal of |
| 2. |
Trichoderma |
Cyclosporin A |
Immunosuppressive |
| 3. |
Monascuspur |
Statins |
Lowering of |
| 4. | Streptococcus | Streptokinase |
Removal of clot |
During sewage treatment, biogases are produced which include :
1. Methane,oxygen, hydrogen sulphide
2. Hydrogen sulphide, methane, sulphur dioxide
3. Methane, hydrogen sulphide, carbon dioxide
4. Hydrogen sulphide, nitrogen, methane
All are correct with respect to BOD (Biochemical oxygen Demand), except
1. It refers to the amount of oxygen that would be consumed if all the organic matter in one liter of water were oxidized by bacteria
2. The BOD test is a measure of the organic matter present in the water
3. The greater the BOD of waste water, less is its polluting potential
4. Waste water is treated till BOD is reduced significantly
The most abundant prokaryotes helpful to humans in making curd from milk and in production of antibiotics are the ones categorized as
1. Chemosynthetic autotrophs
2. Heterotrophic bacteria
3. Cyanobacteria
4. Archaebacteria
What are flocs?
(1) Masses of anaerobic bacteria
(2) Masses of aerobic fungi only
(3) Masses of anaerobic bacteria and fungi
(4) Masses of aerobic bacteria associated with fungal filaments
The most common substrate used in distelleries for the production of ethanol is
(1) Molasses
(2) Corn meal
(3) Soya meal
(4) Ground gram
Select the correct statement from the following
(1) Activated sludge-sediment in settlement tanks of sewage treatment plant is a rich source of aerobic bacteria
(2) Biogas is produced by the activity of aerobic bacteria on animal waste
(3) Methanobacterium is an aerobic bacterium found in rumen of cattle
(4) Biogas, commonly called gobar gas is pure methane
Probiotics are
(1) Live microbial food supplement
(2) Safe antibiotics
(3) Cancer inducing microbes
(4) New kind of food allergens
One of the major difficulties in the biological control of insect/pest is that
(1) The method is less effective as compared with the use of insecticides
(2) The practical difficulty of introducing the predator to specific areas
(3) The predator develops a preference to other deits and may itself become a pest
(4) The predator does not always survive when transferred to a new environment
Which one of the following helps in absorption of phosphorus from soil by plants ?
(1) Anabaena
(2) Glomus
(3) Rhizobium
(4) Frankia
An organism used as a biofertilizer for raising soyabean crop is
1. Nostoc
2. Azobacter
3. Azospirillium
4. Rhizobium
A pollen tube liberates male gametes into
1. Degenerating synergid
2. Intact synergid
3. Antipodals
4. Egg
At what stage of endosperm development will you observe the type of development
1. When divisions start in embryo
2. When embryo is heart shaped
3. Early division of primary endosperm
4. Mature stage of endosperm
Which one of the following is an example of carrying out biological control of pests/diseases using microbes ?
1. Bt- cotton to increase cotton yield
2. Lady bird beetle against aphids in mustard
3. Trichoderma sp. against certain plant pathogens
4. Nucleopolyhedrovirus agains white rust in Brassica
What functions as the embryonic root of the plant?
1. A
2. B
3. C
4. D
The following diagram shows:
(1) Multicarpellary, syncarpus pistil of Papaver
(2) Multicarpellary, apocarpus pistil of Papaver
(3) Multicarpellary, apocarpus gynoecium of Michelia
(4) Multicarpellary, syncarpus gynoecium of Michelia
What is true about the given figure?
| 1. | The plant concerned is Chara, A is Oogonium and B is Antheridium |
| 2. | The plant concerned is Chara, B is Oogonium and A is Antheridium |
| 3. | The plant concerned is Marchantia, A is Oogonium and B is Antheridium |
| 4. | The plant concerned is Marchantia, B is Oogonium and A is Antheridium |
The functions of male sex accessory ducts and glands are maintained by:
| 1. | Hypothalamic releasing hormone |
| 2. | Pituitary gonadotropins |
| 3. | Adrenal cortex steroids |
| 4. | Testicular androgens |
At the birth of the female child, the follicles in her ovaries contain:
| 1. | Primary oocytes that have been arrested at the S phase of the cell cycle |
| 2. | Primary oocytes that have been arrested at the Prophase I of Meiosis I |
| 3. | Secondary oocytes that have been arrested at the Prophase I of Meiosis I |
| 4. | Secondary oocytes that have been arrested at the Metaphase II of Meiosis II |
What happens to the majority of the follicles during the phase from birth to puberty?
1. They get invested by multiple layers of granulosa cells
2. They enlarge in size and then get dormant
3. They undergo atresia or degeneration
4. They cluster together at one end of the ovary
Consider the two surgical procedures given below and choose the correct statement:
| 1. | A is a more difficult procedure than B |
| 2. | The reversibility of A is good but that of B is very poor |
| 3. | A will make the male impotent and B will make the female infertile |
| 4. | Both A and B can be called as sterilization procedures |
In the given diagram, identify A, B, C and D respectively:
| 1. | LH, FSH, Progesterone and Estrogen |
| 2. | FSH, LH, Estrogen and Progesterone |
| 3. | Estrogen, Progesterone, LH and FSH |
| 4. | Progesterone, Estrogen, FSH and LH |
Which of the following is not used as a biopesticide ?
1. Trichoderma harzianum
2. Nuclear Polyhedrosis Virus (NPV)
3. Xanthomonas campestris
4. Bacillus thuringiensis
In the given diagram the part of the female reproductive system that undergoes cyclical changes with changes in the secretion of gonadotropins and gonadal steroids during the menstrual cycle is represented by:
1. A
2. B
3. C
4. D
Which of the structures shown in the following diagram contributes the source of nutrition for the sperms ejaculated in the semen?
(1) A
(2) B
(3) C
(4) D
The vapour pressure of water at 200 C is
17.5. mm Hg. If 18g of glucose ( ) is
added to 178.2 g of water at 200 C, the vapour
pressure of the resulting solution will be
1. 15.750 mm Hg
2. 16.500 mm Hg
3. 17.325 mm Hg
4. 17.675 mm Hg
Two liquids X and Y form an ideal solution.
At 300K, vapour pressure of the solutions
containing 1 mol of X and 3 mol of Y is 550
mmHg. At the same temperature, if 1mol of
Y is further added to this solution, vapour
pressure of the solution increased by
10mmHg. Vapour pressure (in mmHg) of X
and Y in their pure states will be, respectively:
1. 300 and 400
2. 400 and 600
3. 500 and 600
4. 200 and 300
On mixing, heptane and octane to form an
ideal solution. At 373K, the vapour pressures
of the two liquid components (heptane and
octane) are 105 kPa and 45 kPa respectively.
Vapour pressure of the solution obtained by
mixing 25.0g of heptane and 35g of octane
will be (molar mass of heptane and 35g of
octane will be (molar mass of heptane = 100g
mol −1 and of octane = 114g mol −1 )
1. 72.0 kPa
2. 36.1 kPa
3. 96.2 kPa
4. 144.5 kPa
12 g of non volatile solute dissolved in 108g
of water produces the relative lowering of
vapour pressure of 0.1 . The molecular mass
of the solute is
1.80
2.60
3.20
4.40
Kf for water is 1.86K kg mol −1 . If your
automobile radiator holds 1.0kg of water,
how many grams of ethylene glycol
( ) must you add to get the freezing
point of the solution lowered to −2.8°C ?
1. 39g
2. 93g
3. 72g
4. 27g
How many grams of methyl alcohol should
be added to 10 litre tank of water to prevent
it from freezing at 268K?
(Kf for water is 1.86 K Kg mol−1 )
1. 880.07 g
2. 899.04 g
3. 886.02 g
4. 860.2 g
The degree of dissociation (α ) of a weak
electrolyte, is related to van’t Hoff
factor (i) by the expression:
1.
2.
3.
4.
The molar mass of solute X in g mol−1 , if its
1% solution is osmotic with a 5% solution of
cane sugar (molar mass =342 g mol−1 ),is
1. 68.4
2. 34.2
3. 136.2
4. 171.2
The Van't Haff factor of a 0.005 M aqueous solution of KCl is 1.95. The degree of ionisation of KCl is:
1. 0.95
2. 0.97
3. 0.94
4. 0.96
In a face centred cubic lattice, atom A occupies the corner positions and atom B occupies the face
centre positions. If one atom of B is missing from one of the face centred points, the formula of the compound is
1.AB2
2.A2B3
3.A2B5
4.A2B
An aqueous solution of 2% non volatile solute
exerts a pressure of 1.004bar at the normal
boiling point of the solvent.What is the
molecular mass of the solute?
1. 4.135g / mol
2. 22.1g / mol
3. 90.1g / mol
4. 41.35g / mol
Two elements A and B form compounds of
formula AB2 and AB4. When dissolved in 20.0
g of benzene 1.0 g of AB2 lowers F.pt. by 2.30C
whereas 1.0g of AB4 lowers F.pt. by 1.30C.
The Kf for benzene is 5.4. The atomic masses
of A and B are respectively
1. 27, 45
2. 42, 25
3. 52, 48
4. 48, 52
What is the osmotic pressure of the solution obtained by mixing 300cm3 of 2% (mass/volume) solution of urea with 300cm3 of
3.42% (mass/volume) solution of sucrose at 200C ? (R = 0.082L atmK−1mol−1 )
1. 5 atm
2. 5.2 atm
3. 2.6 atm
4. 4.5 atm
Among the electrolytes and , the most effective coagulating agent for sol is
1.
2.
3.
4.
Silver iodide is used for producing artificial
rains because AgI
1. is easy to spray at high altitude
2. is insoluble in water
3. is easy to synthesize
4. has crystals similar to ice
During micelle formation
1. ΔH = + ve, ΔS = + ve
2. ΔH = −ve, ΔS = −ve
3. ΔH = −ve, ΔS = + ve
4. ΔH = + ve, ΔS = −ve
The volume of a colloidal particle, Vc as
compared to the volume of a solute particle
in a true solution Vs could be
1.
2.
3.
4.
The coagulation of 200mL of a positive colloid
took place when 0.73g HCl was added to it
without changing the volume much. The
flocculation value of HCl for the colloid is
1. 0.365
2. 36.5
3. 100
4. 150
Sedimentation potential is the reverse of
1. Electroomosis
2. Electrophoresis
3. Electrokinetic potential
4. Dorn potential
A reaction follows the given concentration (C) vs time graph. The rate for this reaction at 20 seconds will be –
1. 4 × 10–3 Ms–1 2. 8.4 × 10–2 Ms–1
3. 2 × 10–2 Ms–1 4. 7.5 × 10–3 Ms–1
The rate of the simple reaction
2NO+O2 2NO2, when the volume of the reaction vessel is doubled –
1. Will grow eight times of its initial rate
2. Reduce to one-eighth of its initial rate
3. Will grow four times of its initial rate
4. Reduce to one-fourth of its initial rate
For a first order reaction, the plot of 't' against log C gives a straight line with slope equal to–
1.
2.
3.
4. -k
Two substances A (t1/2 = 5min) and (t1/2 = 15 min) are taken in such a way that initially [A] = 4[B]. The time after which both the concentration will be equal is (assuming reactions are of 1st order) :
1. 5 min
2. 15 min
3. 20 min
4. concentration can never be equal
The decomposition of a gaseous substance (A) to yield gaseous products (B) and (C)
follows first order kinetics. If initially only (A) is present and 10 minutes after the
start of the reaction the pressure of (A) is 200 mm Hg and that of over all
mixture is 300 mm Hg, then the rate constant for 2A B + 3 C is –
1. (1/600) ln 1.25 sec–1
2. (2.303/10) log 1.5 min–1
3. (1/10) ln 1.25 sec–1
4. none of these
Under the same reaction conditions, initial concentration of 1.386 mol/dm3 of a substance becomes half in 40 seconds and 20 seconds through first order and zero order kinetics, respectively. Ratio of the rate constant for first order (k1) and zero order (k0) of the reaction is –
1. 0.5 mol–1 dm3 2. 1.0 mol dm–3
3. 1.5 mol dm–3 4. 2 mol–1 dm3
For a first order reaction A P, the temperature (T) dependent rate constant(k) was found to follow the equation logk = – (2000) + 6.0. The pre-exponential factor A and the activation energy Ea,respectively, are-
1. 1.0 × 106 s–1 and 9.2 kJ mol–1
2. 6.0 s–1 and 16.6 kJ mol–1
3. 1.0 × 106 s–1 and 16.6 kJ mol–1
4. 1.0 × 106 s–1 and 38.3 kJ mol–1
Plots showing the variation of the rate constant (k) with temperature (T) are given below. The plot that follows Arrhenius equation is –
1.
2.
3.
4.
The rate of a first order reaction is 0.04 mol litre–1s–1 at 10 minutes and 0.03 mol litre–1
sec–1 at 20 minutes after initiation. Find the half life of the reaction.
1. 24 min
2. 30 min
3. 20 min
4. None of these
Ionic radii of Mg2+ and O2- ions are 66 pm and 140 pm respectively. The type of interstitial void and coordination number of Mg2+ ion respectively are
1. Tetrahedral, 12
2. Octahedral, 6
3. Tetrahedral, 6
4. Octahedral, 8
An element crystallizes in a structure having FCC unit cell of an edge length 200 pm. If
200 g this element contains 24 x 1023 atoms, the density of the element is
1. 50.3 g/cc
2. 63.4 g/cc
3. 41.6 g/cc
4. 34.8 g/cc
Iron has body centred cubic lattice. If the
edge length of the unit cell is 286 pm, the
radius of iron atom is
1. 80 pm 2. 62 pm
3. 160 pm 4. 124 pm
|
|
Column-I |
|
Column-II |
|
A) |
Simple cubic and face-centered cubic |
p) |
Have these cell parameters |
|
B) |
Cubic and rhombohedral |
q) |
Are two crystal systems |
|
C) |
Cubic and tetragonal |
r) |
Have only two crystallographic angles of 90o |
|
D) |
Hexagonal and monoclinic |
s) |
Belong to the same crystal same |
1. A-p,s ; B-p,q; C-q; D-q,r
2. A-p,q ; B-p,s ;C-q,r ;D-p
3. A-q,s; B-r; C-q,r; D-q
4. A-q; B-r; C-p,s; D-q,r
If the radius of octaherdal void is ‘r’ and
radius of the atoms in close packing is ‘R’,
then the relation between ‘r’ and
‘R’ is
1. r = 0.732 R
2. r = 0.414 R
3. r = 0.98 R
4. r = 0.225 R
In a compound, atoms of element Y form
cubical - closest packing and those of element
X occupy 2/3 of tetrahedral voids. The
formula of the compound will be
1. X3Y
2. X4Y3
3. X2Y3
4. X2Y
In a conductivity cell the two platinum electrodes, each of area 10 sq. cm are fixed 1.5 cm apart. The cell contained 0.05 N solution of a salt. If the two electrodes are just half dipped into the solution which has a resistance of 50W, find equivalent conductance of the salt solution –
1. 120 S cm2 eq–1 2. 160 S cm2 eq–1
3. 120 S m2 eq–1 4. 125 S cm2 eq–1
The resistance of a N/10 KCl solution is 245 W. Calculate the equivalent conductance of the solution if the electrodes in the cell are 4 cm apart and each having an area of 7.0 sq. cm -
1. 23.32 S cm2 eq–1 2. 23.23 S m2 eq–1
3. 2.332 S cm2 eq–1 4. None of these
Calculate molar conductivity at infinite dilution of CH3COOH if molar conductivity at infinite dilution of CH3COONa, HCl and NaCl are 91.6, 425.0 and 128.1 S cm2 mol–1 -
1. 390.5 S cm2 mol–1 2. 388.5 S cm2 mol–1
3. 490.5 S cm2 mol–1 4. None of these
The equivalent conductance of an infinitely dilute solution of NH4Cl is 150 and the ionic conductance of OH– and Cl– ions are 198 and 76 respectively . If the equivalent conductance of a 0.01 N solution of NH4OH is 9.6, What will be its degree of dissociation ?
1. 0.0353 2. 0.0103
3. 0.96 4. 0.414
Molar conductance of 0.1 M acetic acid is 7 ohm–1 cm2 mol–1 .
If the molar conductance of acetic acid at infinite dilution is 380.8 ohm–1 cm2 mol–1,
the value of dissociation constant will be -
1. 226 × 10–5 mol dm–3
2. 1.66 × 10–3 mol dm–1
3. 1.66 × 10–2 mol dm–3
4. 3.442 × 10–5 mol dm–3
If is x1, is x2 then will be :
1. 3x2 – 2x1 2. x2 – x1
3. x2 + x1 4. 2x1 + 3x2
The EMF of the cell Ni | Ni2+ || Cu2+ | Cu(s) is 0.59 volt. The standard reduction
electrode potential of copper electrode is 0.34 volt. The standard reduction electrode
potential of nickel electrode will be
1. 0.25 volt 2. – 0.25 volt
3. – 0.50 volt 4. – 0.025 volt
Given that Eº values of Ag+/Ag, K+/K, Mg+2/Mg and Cr+3/Cr are 0.80V, – 2.93V, – 2.37V, and – 0.74 V, respectively. Which of the following orders regarding the reducing power of metal is correct ?
1. Ag > Cr > Mg > K
2. Ag < Cr < Mg < K
3. Ag > Cr > K > Mg
4. Cr > Ag > Mg > K
1/2 H2(g) + AgCl(s) = H+(aq) + Cl–(aq) + Ag(s) occurs in the galvanic cell :
1. Ag/AgCl(s) | KCl(sol) | | AgNO3 (s) | Ag
2. Pt/H2(g) | HCl (sol) | | AgNO3 (s) | Ag
3. Pt/H2(g) | HCl (sol) | | |AgCl(s) | Ag
4. Pt/H2(g) | KCl (sol) | | AgCl(s) | Ag
The half cell reduction potential of a hydrogen electrode at pH = 10 will be :
1. 0.59 V
2. – 0.59 V
3. 0.059 V
4. – 0.059 V
Pt | Cl2 (P1) | HCl (0.1 M) | Pt | Cl2 (P2) ; cell reaction will be spontaneous if :
1. P1 = P2 2. P1 > P2
3. P2 > P1 4. P1 = P2 = 1 atm
Potential in the x-y plane is given as V = 5(x2 + xy) volts. The electric field at the point (1, -2) will be
1. 3 V/m
2. -5 V/m
3. 5 V/m
4. -3 V/m
Two-point charges each of charge +q are fixed at (+a, 0) and (-a, 0). Another positive point charge q placed at the origin is free to move along X-axis. The charge q at origin in equilibrium will have
1. maximum force and minimum potential energy.
2. minimum force and maximum potential energy.
3. maximum force and maximum potential energy.
4. minimum force and minimum potential energy.
A point charge q is placed inside the cavity of a metallic shell. Which one of the diagram correctly represents the electric lines of force ?
1.
2.
3.
4.
The electric field intensity at a point is (20 + 15 )N/C . Considering potential at origin to be zero, the potential at P (2, 4) is
1. -(40 + 60 )V
2. -(10 + 15 )V
3. -100V
4. 20V
A simple pendulum consists of a small sphere of mass m suspended by a thread of length l. The sphere carries a positive charge q. The pendulum is placed in a uniform electric field of strength E directed vertically upwards. With what period will the pendulum oscillate if the electrostatic force acting on the sphere is less than the gravitational force? Assume the oscillations to be small.
1.
2.
3.
4.
A ring has charge Q and radius R. If a charge q is placed at its centre then the increase in tension in the ring is
In a parallel plate capacitor of capacitance C, a metal sheet is inserted between the plates, parallel to them. The thickness of the sheet is half of the separation between the plates. The capacitance now becomes
1. 4C
2. 2C
3. C/2
4. C/4
Two particles A & B are connected by a string such that A is fixed and B is free to oscillate. If A & B both are supplied q charge each, then the ratio of time period of B before and after charging is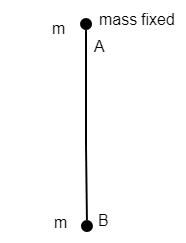
The charge flowing across the cell on closing the key K is equal to-
1. CV
2. CV/2
3. 2CV
4. zero
The conducting spherical shells shown in the figure are connected by a conductor. The capacitance of the system is
A point charge q moves from point P to S along the path PQRS in a uniform electric field pointing parallel to the positive direction of the x-axis. The coordinate of the point P, Q, R and S are (a, b, 0), (2a, 0, 0), (a, -b, 0) and (0, 0, 0) respectively. The work done by the field in the above process is given by the expression
Charge Q is given to the upper plate of an isolated parallel plate capacitor of capacitance C. The potential difference between the plates
Supposing that the earth has a surface charge density of 1 electron/m2; calculate
earth's potential [Given : Radius of Earth = 6400 km]
1. - 0.115Volt
2. - 0.9 Volt
3.- 0.12Volt
4. - 0.10 Volt
A charge Q is distributed over two concentric hollow spheres of radii r and R (R >r) such that the surface densities are equal. Find the potential at the common centre.
1.
2.
3.
4.
Figure shows two conducting thin concentric shells of radii r and 3r. The outer shell carries charge q. Inner shell is neutral. Find the charge that will flow from inner shell to earth after the switch S is closed.
1.
2.
3.
4. None of the above
The equivalent capacitance between A and B is (each of the capacitors obtained is of capacitance equal to C)
1.
2.
3.
4.
An isolated sphere of radius \(R\) contains a uniform volume distribution of positive charge. Which of the curve on the graph below correctly illustrates the dependence of the magnitude of the electric field of the sphere as a function of the distance \(r\) from its centre?

1. A
2. B
3. C
4. D
| 1. | \(26.7\) cm | 2. | \(33.4\) cm |
| 3. | \(46.7\) cm | 4. | \(96.7\) cm |
The current in a wire varies with time according to the equation \(I=(4+2t),\) where \(I\) is in ampere and \(t\) is in seconds. The quantity of charge which has passed through a cross-section of the wire during the time \(t=2\) s to \(t=6\) s will be:
| 1. | \(60\) C | 2. | \(24\) C |
| 3. | \(48\) C | 4. | \(30\) C |
A copper wire is stretched to make it 0.1 % longer. The percentage change in its resistance is
1. 0.2 % increase
2. 0.2% decrease
3. 0.1 % increase
4. 0.1 % decrease
A battery of 10 volt is connected to a resistance of 20 ohm through a variable resistance R. The amount of charge which has passed in the circuit in 4 minutes, if the variable resistance R is increased at the rate of 5 ohm/min.
1.120 coulomb
2.
3.
4.
The potential of point O in the steady state circuit shown is :
1. 11/12V
2. 18/11V
3. 16/9V
4. none of the above
An electrical cable of copper has just one wire of radius 9 mm. Its resistance is 5 . This single wire of cable is replaced by 6 different well-insulated copper wires each of radius 3 mm. The total resistance of the cable will now be equal to?
1. 7.5
2. 45
3. 90
4. 270
A wire 250 cm long and 1 mm2 in cross-section carries a current of 4 A when connected to a 2 V battery. The resistivity of the wire is
1. 0.2 × 10–6 m 2. 2 × 10–6 m
3. 5 × 10–6 m 4. 4 × 10–6 m
The Wheatstone bridge shown in the figure below is balanced when the uniform slide wire \(AB\) is divided as shown. Value of the resistance \(X\) is:

1. \(3~\Omega\)
2. \(4~\Omega\)
3. \(2~\Omega\)
4. \(7~\Omega\)
What is total resistance across terminals \(A\) and \(B\) in the following network?
| 1. | \(R\) | 2. | \(2R\) |
| 3. | \(\dfrac{3R}{5}\) | 4. | \(\dfrac{2R}{3}\) |
There are two points A and B on the extended axis of a 2 cm long bar magnet. Their distances from the centre of the magnet are x and 2x respectively. The ratio of magnetic fields at points A and B will be-
1. 8 : 1 (approximately)
2. 4 : 1 (approximately)
3. 4 : 1
4. 8 : 1
A magnetic material of volume 30 cm3 is placed in a magnetic field of intensity 5 oersted. The magnetic moment produced due to it is 6 amp-m2. The value of magnetic induction will be-
1. 0.2517 Tesla
2. 0.025 Tesla
3. 0.0025 Tesla
4. 25 Tesla.
A circular disc of area \((4 \hat i + 5\hat j) × 10^{ - 3}~\text m^ 2\) is placed in a uniform magnetic field of intensity \((0 . 2 \hat i + 0 . 3 \hat j )~\text T.\) The flux crossing the disc will be:
1. \(23~\text{Wb}\)
2. \(23\times 10^{-2}~\text{Wb}\)
3. \(23\times 10^{-3}~\text{Wb}\)
4. \(23\times 10^{-4}~\text{Wb}\)
Due to a small magnet , intensity at a distance x in the end on position is 9 Gauss. What will be the intensity at same distance in equatorial position ?
1. 9 Gauss
2. 4 Gauss
3. 36 Gauss
4. 4.5 Gauss
A magnet is parallel to a uniform magnetic field. If it is rotated by \(60^{\circ}\), the work done is \(0.8\) J. How much work is done in moving it \(30^{\circ}\) further?
1. \(0.8\times 10^{7}~\text{ergs}\)
2. \(0.4~\text{J}\)
3. \(8~\text{J}\)
4. \(0.8~\text{ergs}\)
A tangent galvanometer shows a deflection 45° when 10 mA current pass through it. If the horizontal component of the earth’s field is and radius of the coil is 10 cm. The number of turns in the coil is
1. 5700 turns
2. 57 turns
3. 570 turns
4. 5.7 turns
The current of 30 A is flowing in a vertical straight wire. If the horizontal component of the earth's magnetic field is 2 x 10-5 Tesla, then the position of the null point will be:
1. 0.9 m
2. 0.3 mm
3. 0.3 cm
4. 0.3 m
A wire of length L carrying current I is bent into a circle of one turn. The field at the center of the coil is B1. A similar wire of length L carrying current I is bent into a square of one turn. The field at its center is B2. Then
1. B1 > B2
2. B1 < B2
3. B1 = B2
4. Nothing can be predicted
An electric current is flowing in a very long pin
as shown in the figure. The value of magnetic
flux density at point O will be-
1.
2.
3.
4.
A long, straight wire is turned into a loop of
radius 10 cm (as shown in figure). If a current of
8 ampere is passed through the loop, then the
value of the magnetic field B at the centre C of
the loop will be (Wb/m2)-
1. 3.424 × 10–5, vertically upward
2. 3.424 × 10–5 , vertically downward
3. 4.24 × 10–5 , vertically upward
4. 4.24 × 10–5, vertically downward
A 6.28m long wire is turned into a coil of diameter
0.2m and a current of 1 amp. is passed in it. The
magnetic induction at its centre will be-
1. 6.28 × 10-5 Tesla
2. 0
3. 6.28 Tesla
4. 6.28 × 10-3 Tesla
An particle is moving in a magnetic field of
( ) tesla with a velocity of 5×105 m/s. The
magnetic force acting on the particle will be-
1. 3.2 × 10–13 dyne
2. 3.2 × 1013 N
3. 0
4. 3.2 × 10–13 N
A potential difference of 600 volts is applied across the plates of a parallel plate condenser placed in a magnetic field. The separation between the plates is 3 mm. An electron projected vertically upward parallel to the plates with a velocity of 2 × 106 m/s moves undeflected between the plates. The magnitude and direction of the magnetic field in the region between the condenser plates will be (in Wb/) (Given charge of electron = –1.6 × 10–19 coulomb).
1. 0.1, into the plane.
2. 0.2, into the plane.
3. 0.3, out of the plane.
4. 0.4, out of the plane.
An -particle is describing a circle of radius 0.45
m in a field of magnetic induction 1.2 weber/m2.
The potential difference required to accelerate
the particle, so as to give this much energy to it
(The mass of -partical is 6.8 × 10-27 kg and its
charge is 3.2 × 10–19 coulomb.) will be-
1. 6 × 106 V
2. 2.3 × 10–12 V
3. 7 × 106 V
4. 3.2 × 10–12 V
A straight horizontal stretch of copper wire
carries a current i = 30 A. The linear mass density
of the wire is 45 g/m. What is the magnitude of
the magnetic field needed to "float" the wire,
that is to be balance its weight?
1. 147 G 2. 441 G
3. 14.7 G 4. 0 G
A loop of flexible conducting wire of length
0.5 m lies in a magnetic field of 1.0 T perpendicular
to the plane of the loop. The tension developed
in the wire if the current is of 1.57A. will be-
1. 0.15 N 2. 0.25 N
3. 0.125 N 4. 0.138 N
An ammeter and a voltmeter are connected in series to a battery with an emf E = 6 volt . When a certain resistance is connected in parallel with voltmeter, the reading of latter decreases two times, where as the reading of the ammeter increasing the same number of times. What is ratio of resistance of voltmeter to resistance of ammeter?
1. 2 2. 1/2
3. 1/3 4. 3
An ammeter and a voltmeter are connected in series to a battery of an emf E = 6 volt. When a certain resistance is connected in parallel with voltmeter, the reading of voltmeter decreases by two times, where as the reading of the ammeter increases by the same number of times. What would be voltmeter reading before connecting the resistance ?
1. 1 V 2. 2 V
3. 3 V 4. 4 V